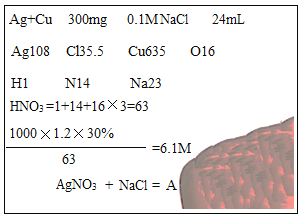
��Ŀ����
����Ŀ���������ƣ�CaO2���������о��й㷺��Ӧ��
��һ���������Ƶ���������;
��1��CaO2����ϡ���ᷢ�����ֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ_____��
��2�����;���������������Ҫ�ɷ�ΪCaO2������ˮ������Ӧ����O2��������һ�ּ�仯ѧʽΪ_____��Na2O2Ҳ����ˮ��Ӧ��ԭ����CaO2��ͬ����ȴ������Ϊ��Ϻ����Ĺ���������������ܵ�ԭ��_____��
�������������ƾ�����Ʊ�
�����ϣ��������ƾ���(CaO2��yH2O)������Ϊ��ɫ���������ᣬ�����ھƾ���
�Ʊ�ԭ����CaCl2��H2O2��NH3��H2O��CaO2��yH2O����NH4Cl��װ�����¡�
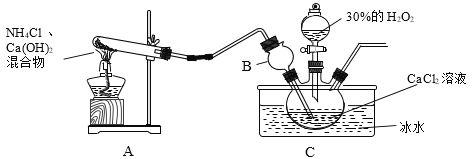
��1��װ��A���Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____��
��2��װ��C���ñ�ˮԡ�����¶���0�����ң����ܵ�ԭ����Ҫ�У�
��.�÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��yH2O���ʣ�
��._____��
��3����Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2��yH2O��
��ϴ��ʱ����95%�ľƾ���Һϴ�ӵ��ŵ���_____��
�ڼ��龧����ϴ�Ӹɾ��ķ���Ϊ_____��
�������������ƾ�����ɵIJⶨ
��ȡ21.6�˾������ȷ����Ƕ�������ȷֽ�ʵ�飬�����Ƴɹ����������¶ȹ�ϵͼ���������ƾ�������ʱ����ʧȥ�ᾧˮ����
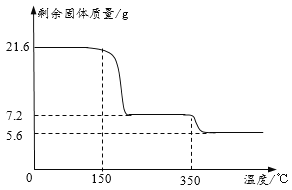
��1��0��150�������������ı��ԭ����_____��
��2��������ͼ��֪y=_____����д��������̣�
��3��350��ʱ������Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡�CaO2+2HCl=H2O2+CaCl2 Ca(OH)2 ��Ӧ�����������ƣ�����ǿ��ʴ�� Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O ��ֹ��������ֽ� ���پ�����ʧ�����ڸ��� ȡ���һ��ϴ�����õ���Һ�����Թ��У�����AgNO3��Һ����������ϴ�� δ�ﵽ��Ӧ������¶� 8 2CaO2
CaCl2+2NH3��+2H2O ��ֹ��������ֽ� ���پ�����ʧ�����ڸ��� ȡ���һ��ϴ�����õ���Һ�����Թ��У�����AgNO3��Һ����������ϴ�� δ�ﵽ��Ӧ������¶� 8 2CaO2![]() 2CaO+O2��
2CaO+O2��
��������
��һ����1��CaO2��HCl��Ӧ����H2O2��CaCl2������ʽ�ǣ�CaO2+2HCl=H2O2+CaCl2��
��2�����������غ㶨�ɿ�֪�������к��и�Ԫ�أ�����CaO2��ˮ������Ӧ�����������������ƣ�Na2O����ˮ��Ӧ�������������������ƣ�����������ǿ���ԣ���������Ϻ���森���ԣ�Na2O2������Ϊ��Ϻ����Ĺ�������
��������1���������ƺ��Ȼ���ڼ��������·�Ӧ�����Ȼ��ơ�������ˮ�����Է���ʽ�ǣ�Ca��OH��2+2NH4Cl![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��2��װ��C���ñ�ˮԡ�����¶���0�����ң���һ�����ܵ�ԭ����Ҫ���¶ȸ�ʱ�����������ֽ�����ˮ��������
��3���ٹ������ƾ��壨CaO2yH2O���������ھƾ����ƾ��ӷ�������ϴ��ʱ����95%�ľƾ���Һϴ�ӵ��ŵ��Ǽ��پ�����ʧ�����ڸ�����������ϴ�Ӳ��ɾ���������Ȼ�泥������������ӣ�����ȡ���һ��ϴ�����õ���Һ�����Թ��У���������������а�ɫ����������˵��û��ϴ�ɾ������û������˵��ϴ�ɾ��ˣ�
��������1��0��150�������������ı��ԭ����δ�ﵽ��Ӧ������¶ȡ�
��2���������ƾ�������ʱ����ʧȥ�ᾧˮ����ʧȥ��ˮ������Ϊ��21.6g-7.2g=14.4g���ʹ������ƺ�ˮ�ķ��Ӹ�����Ϊ��![]() =1��8����y=8��
=1��8����y=8��
��3��350��ʱ��ȫ�ֽ�ʣ���������5.6g�����ٵ�����Ϊ7.2g-5.6g=1.6g���ʼ��ٵ�����Ԫ�أ���ɵ��������������ķֽⷴӦ����ʽΪ��2CaO2![]() 2CaO+O2����
2CaO+O2����

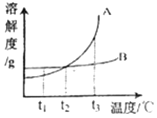
����Ŀ��������Ȼ��ƺ�̼�����ڲ�ͬ�¶�ʱ���ܽ�ȣ����ݴ˱��ش�
�¶�/�� | 10 | 20 | 30 | 40 | |
�ܽ��/g | �Ȼ��� | 35.8 | 36.0 | 36.3 | 36.6 |
̼���� | 12.2 | 21.8 | 39.7 | 53.2 | |
��1��40��ʱ���Ȼ��Ƶ��ܽ��Ϊ_____g��
��2����20��ʱ����30g̼���Ƽ���100gˮ�У���ֽ����õ���Һ��������������Ϊ_____����������ȷ��0.1%��
��3��10��ʱ���ֱ�ȡ���������Ȼ��ƺ�̼�������Ʊ�����Һ����Ҫˮ�������Ȼ���_____���������������������̼���ƣ�