��Ŀ����
�Ͼ�ij��ѧѧϰС�鿼����ܶ���Ϊ�ܶ�����̬�����ʯ�������ʯ����̾��������ƺ�ʵʩ��ʵ��--ģ���ܶ��ġ��γɡ����ӽ̲��ϲ���������ϣ�
�ܶ����ֲ���ʯ������ɵ�ɽ���У�ʯ���ҵ���Ҫ�ɷ���̼��ƣ����������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O�TCa��HCO3��2������̼����Ƶ�ˮ���Ȼ�ѹǿͻȻ��Сʱ���ܽ���ˮ���̼����ƾͻ�ֽ⣬��������̼��Ƴ���������ͬʱ�ų�������̼��Ca��HCO3��2�TCaCO3��+CO2��+H2O��������ˮ������������ʱ��ˮ�е�̼����Ʒ���������Ӧ���еij����ڶ������еij����ڶ��ף��ջ����ۣ��������γ�����ʯ�������γ�ʯ������ʯ��ʯ������ʱ���γ���ʯ����
��1��������Ʋ������ģ���ܶ����γɡ���ʵ�飮��ʵ�������ͼ���Իش�
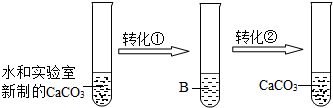
ʵ��ת������Ҫͨ������������A��A�Ļ�ѧʽ��______��B��Һ�����ʵĻ�ѧʽ��______��ʵ��ת���ڣ�ͨ�����õĻ�������������______��
��2�������òɼ���ʯ������Ʒ�������ʵ�飮���ɼ�������Ʒ��ˮ��ϴ�����ɣ���ȡ24.00g��Ʒƽ���ֳ����ݣ��ֱ���������ͬ����������ϡ���ᷴӦ�������вⶨ����ͼ1���������ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵͼ����ͼ2����
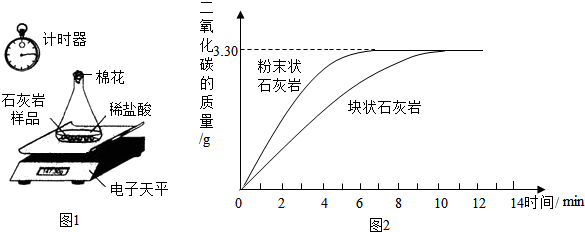
��ͼ1��ʾʵ�������ݼ�¼ֽ��Ӧ��������¼��ʵ��������______��______��
����ͼ2�����߿��Կ���������������Һ�����ʷ�Ӧ��������������ͬʱ���Ӵ����Խ______���䷴Ӧ����Խ______��
��������Ʒ��̼��Ƶ�����������������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ�����
�ܶ����ֲ���ʯ������ɵ�ɽ���У�ʯ���ҵ���Ҫ�ɷ���̼��ƣ����������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O�TCa��HCO3��2������̼����Ƶ�ˮ���Ȼ�ѹǿͻȻ��Сʱ���ܽ���ˮ���̼����ƾͻ�ֽ⣬��������̼��Ƴ���������ͬʱ�ų�������̼��Ca��HCO3��2�TCaCO3��+CO2��+H2O��������ˮ������������ʱ��ˮ�е�̼����Ʒ���������Ӧ���еij����ڶ������еij����ڶ��ף��ջ����ۣ��������γ�����ʯ�������γ�ʯ������ʯ��ʯ������ʱ���γ���ʯ����
��1��������Ʋ������ģ���ܶ����γɡ���ʵ�飮��ʵ�������ͼ���Իش�

ʵ��ת������Ҫͨ������������A��A�Ļ�ѧʽ��______��B��Һ�����ʵĻ�ѧʽ��______��ʵ��ת���ڣ�ͨ�����õĻ�������������______��
��2�������òɼ���ʯ������Ʒ�������ʵ�飮���ɼ�������Ʒ��ˮ��ϴ�����ɣ���ȡ24.00g��Ʒƽ���ֳ����ݣ��ֱ���������ͬ����������ϡ���ᷴӦ�������вⶨ����ͼ1���������ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵͼ����ͼ2����

��ͼ1��ʾʵ�������ݼ�¼ֽ��Ӧ��������¼��ʵ��������______��______��
����ͼ2�����߿��Կ���������������Һ�����ʷ�Ӧ��������������ͬʱ���Ӵ����Խ______���䷴Ӧ����Խ______��
��������Ʒ��̼��Ƶ�����������������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ�����
��1����ͼ�в����Ե�̼��ƾ�ת���ٱ�ɿ����Եģ��������е���Ϣ��ʯ���ҵ���Ҫ�ɷ���̼��ƣ����������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O�TCa��HCO3��2����֪����A�Ƕ�����̼���仯ѧʽΪCO2��B�е�������̼����ƣ��仯ѧʽΪCa��HCO3��2��
��ͼ�п����Ե�̼����ƾ�ת���ڱ�ɲ����Ե�̼��ƣ����������ṩ����Ϣ������̼����Ƶ�ˮ���Ȼ�ѹǿͻȻ��Сʱ���ܽ���ˮ���̼����ƾͻ�ֽ⣬��������̼��Ƴ���������ͬʱ�ų�������̼��Ca��HCO3��2�TCaCO3��+CO2��+H2O����֪��ʵ����һת���ķ����м��Ȼ��ѹ���������м�����ͨ�����õĻ�������������
��2���ٽ��ͼ2��ʾ����������֪ͼ1��ʵ�������ݼ�¼ֽ��Ӧ��������¼��ʵ�������ǵ�����ƽʾ��������ƿ��ϵ�������ͼ�ʱ��ʾ������Ӧʱ�䣩��
����ͼ2��ʾ��ͼ���֪���ڷ�Ӧ����ǰijһ�¶�ʱ����ĩ״��ʯ���Ҳ����Ķ�����̼�࣬�����ڷ�ĩ״��ʯ������ϡ����ĽӴ��������˷�Ӧ���ʿ죻��״��ʯ���Ҳ����Ķ�����̼�٣������ڿ�״��ʯ������ϡ����ĽӴ����С����˷�Ӧ��������
����ÿ����Ʒ��CaCO3������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.30g
=
��
x=7.50g
��Ʒ��̼��Ƶ���������Ϊ
��100%=62.5%
�ʴ�Ϊ��
��1��CO2��Ca��HCO3��2������
��2���ٵ�����ƽʾ��������ƿ��ϵ����������ʱ��ʾ������Ӧʱ�䣩���𰸲����Ⱥ�˳��
�ڴ�죨��С����
����Ʒ��̼��Ƶ���������Ϊ62.5%
��ͼ�п����Ե�̼����ƾ�ת���ڱ�ɲ����Ե�̼��ƣ����������ṩ����Ϣ������̼����Ƶ�ˮ���Ȼ�ѹǿͻȻ��Сʱ���ܽ���ˮ���̼����ƾͻ�ֽ⣬��������̼��Ƴ���������ͬʱ�ų�������̼��Ca��HCO3��2�TCaCO3��+CO2��+H2O����֪��ʵ����һת���ķ����м��Ȼ��ѹ���������м�����ͨ�����õĻ�������������
��2���ٽ��ͼ2��ʾ����������֪ͼ1��ʵ�������ݼ�¼ֽ��Ӧ��������¼��ʵ�������ǵ�����ƽʾ��������ƿ��ϵ�������ͼ�ʱ��ʾ������Ӧʱ�䣩��
����ͼ2��ʾ��ͼ���֪���ڷ�Ӧ����ǰijһ�¶�ʱ����ĩ״��ʯ���Ҳ����Ķ�����̼�࣬�����ڷ�ĩ״��ʯ������ϡ����ĽӴ��������˷�Ӧ���ʿ죻��״��ʯ���Ҳ����Ķ�����̼�٣������ڿ�״��ʯ������ϡ����ĽӴ����С����˷�Ӧ��������
����ÿ����Ʒ��CaCO3������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.30g
| 100 |
| 44 |
| x |
| 3.30g |
x=7.50g
��Ʒ��̼��Ƶ���������Ϊ
| 7.50g |
| 24g��2 |
�ʴ�Ϊ��
��1��CO2��Ca��HCO3��2������
��2���ٵ�����ƽʾ��������ƿ��ϵ����������ʱ��ʾ������Ӧʱ�䣩���𰸲����Ⱥ�˳��
�ڴ�죨��С����
����Ʒ��̼��Ƶ���������Ϊ62.5%

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ