��Ŀ����
��7�֣���֪A~G�������ʶ��dz��л�ѧ�α��г��ֹ��Ļ��������F�dz��õĽ������ϣ�HΪ�����ĵ��ʡ�������֮���������ת����ϵ����Ӧ��������ͼ��ʾ����
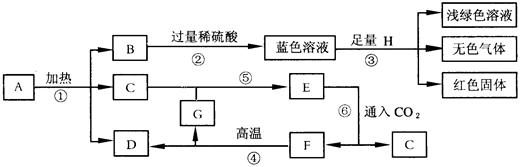
��1����д������A�Ļ�ѧʽ ��������ɫ��Һ�е������� �� ��
��2���ڢ�~��Ӧ�����ڻ��Ϸ�Ӧ�ķ���ʽ�� ��
��3������ת�������У����ܹ���ӳ�ҹ��Ŵ������ڻ�ѧ�������������ΰ���ı仯��
��ٳ�����һ�����û�ѧ����ʽ��ʾ ��

��1����д������A�Ļ�ѧʽ ��������ɫ��Һ�е������� �� ��
��2���ڢ�~��Ӧ�����ڻ��Ϸ�Ӧ�ķ���ʽ�� ��
��3������ת�������У����ܹ���ӳ�ҹ��Ŵ������ڻ�ѧ�������������ΰ���ı仯��
��ٳ�����һ�����û�ѧ����ʽ��ʾ ��
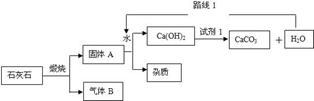
��1��Cu2(OH)2CO3 ��CuSO4��H2SO4 ��
��2��CaO + H2O = Ca(OH)2 ��
��3��CaCO3���� CaO + CO2�� ��Fe + CuSO4 = FeSO4 + Cu ��
��2��CaO + H2O = Ca(OH)2 ��
��3��CaCO3���� CaO + CO2�� ��Fe + CuSO4 = FeSO4 + Cu ��
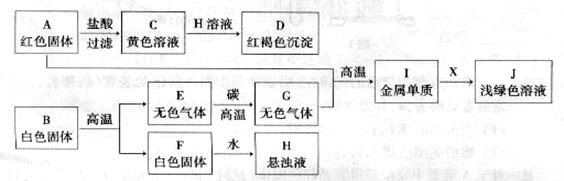
����F�dz��õĽ������ϣ�����FΪ̼��ƣ�̼��Ƹ������������ơ�������̼��
B�����ϡ���ᷴӦ���õ���ɫ��Һ��������ɫ��Һ��ͭ���ӵ���Һ��������ɫ��Һ������ͭ��Һ�����滹���й�����ϡ���ᣮH�ǵ��ʲ�������ɫ��Һ��Ӧ������dz��ɫ��Һ����ɫ���塢��ɫ���壬����H������dz��ɫ��Һ��������������ɫ��������������ɫ������ͭ��
����B��������ᷴӦ��������ͭ������B������ͭ����Eͨ�������̼����̼��ƣ��Ƴ�E���������ƣ�C��ˮ������D���Ƕ�����̼����ΪA������������ͭ��ˮ��������̼������A�Ǽ�ʽ̼��ͭ��
B�����ϡ���ᷴӦ���õ���ɫ��Һ��������ɫ��Һ��ͭ���ӵ���Һ��������ɫ��Һ������ͭ��Һ�����滹���й�����ϡ���ᣮH�ǵ��ʲ�������ɫ��Һ��Ӧ������dz��ɫ��Һ����ɫ���塢��ɫ���壬����H������dz��ɫ��Һ��������������ɫ��������������ɫ������ͭ��
����B��������ᷴӦ��������ͭ������B������ͭ����Eͨ�������̼����̼��ƣ��Ƴ�E���������ƣ�C��ˮ������D���Ƕ�����̼����ΪA������������ͭ��ˮ��������̼������A�Ǽ�ʽ̼��ͭ��

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
�����Ŀ