��Ŀ����
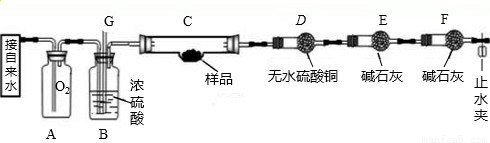
ij��ѧ��ȤС����һ����̼���������ķ�Ӧ��̽��������ԭ����װ������ͼa��ʾ����ش��й�����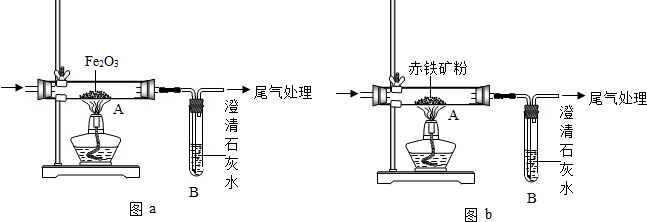
��1��ʵ�鿪ʼʱ��Ӧ
��2��ʵ�����һ��ʱ�������A�г��ֵ�����Ϊ
��3����һ�������£���CO�⣬������Щ���ʿ��Ի�ԭFe2O3
��4��Ϊ�ⶨij���������Ʒ��Fe2O3 ������������������ʯ����Ҫ�ɷ���Fe2O3��������ȤС������ͼbװ�ã����������ʵ�鷽����װ�����������ã�������������ʲ����뷴Ӧ��������������Ʒ�е�Fe2O3��ȫ��Ӧ����
��ȡ������Ʒ����������Ʒ�������� �ڲ����Ӧǰ�Թ�B����������������
�۲����Ӧ���Թ�B���������������� �ܼ�������������Ʒ��Fe2O3������������
ijͬѧ��Ϊ����ʵ�鷽������һ����ȷ������������Ʒ��Fe2O3������������������
���ı�װ�ú�ҩƷ���㻹����ͨ���ⶨ
��5��Ϊ��ֹʵ����β���Դ�������Ⱦ����д������β����һ�ַ���
��������1����ȼ�Ե��������ȼ�Ե������� ��ȼʱ�ᷢ����ը��
��2�����ݷ�Ӧ���������Լ���Ӧ����ȷ������ʽ����Ӧ����
��3�����л�ԭ�Ե�������������̼��һ����̼��
��4��Ҫ�ⶨ������Fe2O3���������������Ը������ɵĶ�����̼���������Ƿ�Ӧǰ��������ʵ������������м��㣮
��5��һ����̼�ж��������β��������
��2�����ݷ�Ӧ���������Լ���Ӧ����ȷ������ʽ����Ӧ����
��3�����л�ԭ�Ե�������������̼��һ����̼��
��4��Ҫ�ⶨ������Fe2O3���������������Ը������ɵĶ�����̼���������Ƿ�Ӧǰ��������ʵ������������м��㣮
��5��һ����̼�ж��������β��������
����⣺��1��һ����̼���п�ȼ�Ժ���������ڵ�ȼ�������»ᷢ����ը���ʴ�Ϊ����ͨCO�ټ��ȡ��ž�װ���ڵĿ�������ֹ����ʱ������ը��
��2��һ����̼��ԭ�������ڼ��ȵ��������������Ͷ�����̼���������Ǻ�ɫ��ĩ�����Ǻ�ɫ�ķ�ĩ���ʴ�Ϊ������ɫ������ɺ�ɫ��3CO+Fe2O3
2Fe+3CO2��
��3��H2��C��һ����̼�����л�ԭ�ԣ��ʴ�Ϊ��H2��C��
��4��ʯ��ˮ���������Ƶ�ˮ��Һ����������������ˮ�����ʣ��������������٣���һ���ܽ����ɵĶ�����̼ȫ�����գ����Բ�һ����ȷ���������Fe2O3���������������������ʵ��ͨ���ⶨ��Ӧǰ��������ʵ���������м��㣻�ʴ�Ϊ�����ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ���ա��������ơ���Ӧǰ����������Ʒ�����ͷ�Ӧ��������ʣ����������
��5������һ����̼�ж����������β������������һ����̼�����ʿ�ȷ��β�������ķ������ʴ�Ϊ����ȼ���ռ�
��2��һ����̼��ԭ�������ڼ��ȵ��������������Ͷ�����̼���������Ǻ�ɫ��ĩ�����Ǻ�ɫ�ķ�ĩ���ʴ�Ϊ������ɫ������ɺ�ɫ��3CO+Fe2O3
| ||
��3��H2��C��һ����̼�����л�ԭ�ԣ��ʴ�Ϊ��H2��C��
��4��ʯ��ˮ���������Ƶ�ˮ��Һ����������������ˮ�����ʣ��������������٣���һ���ܽ����ɵĶ�����̼ȫ�����գ����Բ�һ����ȷ���������Fe2O3���������������������ʵ��ͨ���ⶨ��Ӧǰ��������ʵ���������м��㣻�ʴ�Ϊ�����ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ���ա��������ơ���Ӧǰ����������Ʒ�����ͷ�Ӧ��������ʣ����������
��5������һ����̼�ж����������β������������һ����̼�����ʿ�ȷ��β�������ķ������ʴ�Ϊ����ȼ���ռ�
���������⿼����ǽ�������ұ�����������ݿα�����֪ʶ�����ش����漰���������������ĺ����ⶨʱ������ע����������ȷ�ԣ��ų��������أ�

��ϰ��ϵ�д�
�����Ŀ