��Ŀ����
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷֽ���������̽������������»���ش�������⣮
���������ϡ���Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������--ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���20�棩��
| ������ ������ | OH- | CO32- | HCO3- |
| Ca2+ | �� | ���� | ���� |
| Mg2+ | ���� | �� | ���� |
��������⡿ˮ������Ҫ�ɷ����Ƿ���Ca��OH��2��MgCO3�أ�
��ʵ�鷽��1��ȷ��ˮ�����Ƿ�Ca��OH��
| ʵ�鲽�� | ʵ������ | ���� |
| �����������ˮ���У���������������ˮ��ֽ��裬���ˣ�����Һ�����Na2CO3��Һ�� | û�а�ɫ�������� | ˮ����______�� |
������ͼ��ʾ��ʵ��װ�ã����ʵ��2̽��������Ҫʵ�鲽�����£�
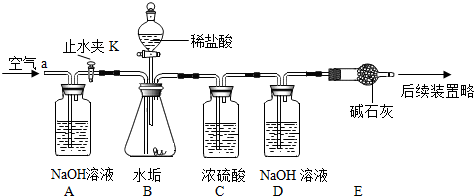
��ȷ����D��Eװ��������Ϊ600.0g������ͼ��װ��9.8gˮ������������ƿ�У���������ϡ������Һ������ƿ�в��ٲ�������ʱ����ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ604.4g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������ϡ����Ļӷ����Լ�װ���ڿ�������������ʵ���Ӱ�죩��
��ʵ�����ۡ�
��1������ҩƷǰӦ______����Ӧ�������ֹˮ��K���������������Ŀ����______��
��2����дһ��װ��B����������Ļ�ѧ��Ӧ����ʽ��______��
��3��װ�� B������CO2������Ϊ______g��ͨ������˵����ˮ����______������ţ���MgCO3��
A��һ���������� B��һ�������������� C�����ܡ������� D����ȷ��
��ʵ�鷽��3������̽��ˮ����������þ����������
��ȡ����Ϊ9.8g��ˮ������������7.3%ϡ������֮ǡ����ȫ��Ӧ���������ϡ����110g������������ݺ�ʵ����ۣ�����ˮ����Ʒ��������þ������������д�������ļ�����̣�����������һλС������
�⣺�������ϣ����ݱ�����Կ�����̼��ƺ�������þ�Dz�����ˮ�����ʣ���ˮ����һ������̼��ƺ�������þ�����CaCO3��Mg��OH��2��
ʵ�鷽��������Һ�����Na2CO3��Һ��û�в�����ɫ������˵���������������ƣ��������Ca��OH��2��
ʵ�����ۣ���1����Ϊʵ����Ҫ�ⶨ���ɵĶ�����̼����������Ҫ��֤װ�ò�©����������Ҫ���װ�õ������ԣ���Ӧ�������ֹˮ��K���������������Ϊ�˽�װ���еĶ�����̼ȫ�����գ�������װ�õ������ԣ���������װ���еĶ�����̼ȫ������D��Eװ���г�����գ�
��2��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ӧǰ��D��Eװ�õ�������Ϊ��604.4g-600g=4.4g��������4.4g������̼��Ҫ̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
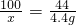 x=10g����ˮ��������Ϊ9.8g����һ�����������ɶ�����̼��̼��þ���ڣ�
x=10g����ˮ��������Ϊ9.8g����һ�����������ɶ�����̼��̼��þ���ڣ�
���4.4��A��
ʵ�鷽��3���⣺�����ڲ���������̼����������Ϊx��������þ����Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2��
73 44
x��7.3% 4.4g
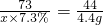 x=100g
x=100g
Mg��OH��2+2HCl�TMgCl2+2H2O
58 73
y 10g��7.3%
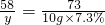 y=0.58g
y=0.58g
������þ������������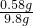 ��100%��5.9%
��100%��5.9%
��ˮ����Ʒ��������þ����������Ϊ5.9%��
�������������ϣ��������ʵ�ˮ���Խ��з�����
ʵ�鷽�������������������������̼���η�Ӧ���ɳ������з�����
ʵ�����ۣ�Ҫ�ⶨ������̼�����������뱣֤װ�����������ã����Ը��ݷ�Ӧǰ��װ��D��E��������ȷ�����ɵĶ�����̼������������ȷ��ˮ�����Ƿ���̼��þ����������õ�����������������Ӷ���������Ƿ���������þ��
���������⿼���˳������ʳɷֵ��ƶϣ���ɴ��⣬�����������ʵ����ʽ������ṩ����Ϣ���У�
ʵ�鷽��������Һ�����Na2CO3��Һ��û�в�����ɫ������˵���������������ƣ��������Ca��OH��2��
ʵ�����ۣ���1����Ϊʵ����Ҫ�ⶨ���ɵĶ�����̼����������Ҫ��֤װ�ò�©����������Ҫ���װ�õ������ԣ���Ӧ�������ֹˮ��K���������������Ϊ�˽�װ���еĶ�����̼ȫ�����գ�������װ�õ������ԣ���������װ���еĶ�����̼ȫ������D��Eװ���г�����գ�
��2��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ӧǰ��D��Eװ�õ�������Ϊ��604.4g-600g=4.4g��������4.4g������̼��Ҫ̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
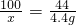 x=10g����ˮ��������Ϊ9.8g����һ�����������ɶ�����̼��̼��þ���ڣ�
x=10g����ˮ��������Ϊ9.8g����һ�����������ɶ�����̼��̼��þ���ڣ����4.4��A��
ʵ�鷽��3���⣺�����ڲ���������̼����������Ϊx��������þ����Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2��
73 44
x��7.3% 4.4g
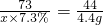 x=100g
x=100gMg��OH��2+2HCl�TMgCl2+2H2O
58 73
y 10g��7.3%
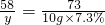 y=0.58g
y=0.58g ������þ������������
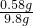 ��100%��5.9%
��100%��5.9% ��ˮ����Ʒ��������þ����������Ϊ5.9%��
�������������ϣ��������ʵ�ˮ���Խ��з�����
ʵ�鷽�������������������������̼���η�Ӧ���ɳ������з�����
ʵ�����ۣ�Ҫ�ⶨ������̼�����������뱣֤װ�����������ã����Ը��ݷ�Ӧǰ��װ��D��E��������ȷ�����ɵĶ�����̼������������ȷ��ˮ�����Ƿ���̼��þ����������õ�����������������Ӷ���������Ƿ���������þ��
���������⿼���˳������ʳɷֵ��ƶϣ���ɴ��⣬�����������ʵ����ʽ������ṩ����Ϣ���У�

��ϰ��ϵ�д�
�����Ŀ
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷֽ���������̽������������»�����ش�������⣮
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3_�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������--ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
[�������]ˮ������Ҫ�ɷ���һ������ ���ѧʽ����ͬ���� �����ܺ���Ca��OH��2��MgCO3��
[��Ʒ���]ʵ��1��ȷ��ˮ�����Ƿ�Ca��OH��2
ʵ��2��ȷ��ˮ�����Ƿ�MgCO3
��2.50gˮ����Ʒ������ƿ�У�����������ϡ���ᣬ�������ɵĶ�����̼����1.25g��
��ش�
��1��д��̼��������ᷴӦ�Ļ�ѧ����ʽ ��
��2����ʽ���㣬���ˮ����û��̼��þ����̼��Ƶ�����Ϊ���٣�
��3��ˮ���� ��MgCO3������ĸ����
A��һ�� B��һ���� C������ D����ȷ����
�����ǣ� ��
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3_�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������--ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
| ���ָơ�þ�������ܽ��Ա���20�棩 | |||
| OH- | CO32- | HCO3- | |
| Ca2+ | �� | ���� | ���� |
| Mg2+ | ���� | �� | ���� |
[��Ʒ���]ʵ��1��ȷ��ˮ�����Ƿ�Ca��OH��2
| ʵ�鲽�� | ʵ������ | �йػ�ѧ��Ӧ����ʽ |
| �����������ˮ���м�������������ˮ��ֽ��裬���ˣ�����Һ�м���Na2CO3��Һ�� | �а�ɫ�������� |
��2.50gˮ����Ʒ������ƿ�У�����������ϡ���ᣬ�������ɵĶ�����̼����1.25g��
��ش�
��1��д��̼��������ᷴӦ�Ļ�ѧ����ʽ
��2����ʽ���㣬���ˮ����û��̼��þ����̼��Ƶ�����Ϊ���٣�
��3��ˮ����
A��һ�� B��һ���� C������ D����ȷ����
�����ǣ�
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷݽ���������̽������������»�����ش�������⣮
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������----ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
���ָơ�þ�������ܽ��Ա���20�棩
[�������]
����һ��ˮ���ijɷ���______
�������ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2
��������ˮ���ijɷ���CaCO3��Mg��OH��2��MgCO3
�����ģ�ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2 MgCO3
[�������]
��1��ȷ��ˮ�����Ƿ����������ƣ�2��ȷ��ˮ�����Ƿ���̼��þ
[ʵ�鲽��]
��1��
��2��������ͼʵ��װ����ɣ�2����̽��������Ҫʵ�鲽�����£�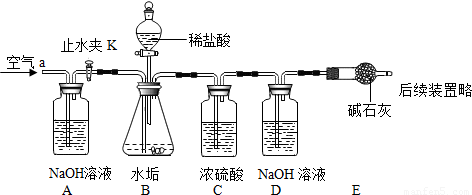
����D��Eװ��������Ϊ400.00g
����ͼ��װ��3gˮ����Ʒ������ƿ�У���������ϡ��������ƿ�в��ٲ������ݣ��رշ�Һ©������
��ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ401.54g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
����ʵ��ش��������⣺
�ټ�����Ʒǰ��Ӧ______��װ��C��������______����Ӧ�������ֹˮ��K���������������Ŀ����______
��װ��D�еĻ�ѧ����ʽΪ______
��װ��B������CO2������Ϊ______g��ˮ����______��MgCO3��
A��һ�� B��һ���� C������ D����ȷ��
[��˼����]
���֤��ˮ����ȷʵ��������þ��С��������Լ����뷨��
��ȡ����Ϊ3g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����Wg���������ʵ����ۣ��ж�W��ֵ����______����ʱ��ˮ����һ������Mg��OH��2��
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������----ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
���ָơ�þ�������ܽ��Ա���20�棩
| ������ ������ | OH- | CO32- | HCO3- |
| Ca2+ | �� | ���� | ���� |
| Mg2+ | ���� | �� | ���� |
����һ��ˮ���ijɷ���______
�������ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2
��������ˮ���ijɷ���CaCO3��Mg��OH��2��MgCO3
�����ģ�ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2 MgCO3
[�������]
��1��ȷ��ˮ�����Ƿ����������ƣ�2��ȷ��ˮ�����Ƿ���̼��þ
[ʵ�鲽��]
��1��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �����������ˮ���м�������������ˮ��ֽ��裬���ˣ�����Һ�м���______��Һ | ��______���� | ˮ������ �������� |

����D��Eװ��������Ϊ400.00g
����ͼ��װ��3gˮ����Ʒ������ƿ�У���������ϡ��������ƿ�в��ٲ������ݣ��رշ�Һ©������
��ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ401.54g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
����ʵ��ش��������⣺
�ټ�����Ʒǰ��Ӧ______��װ��C��������______����Ӧ�������ֹˮ��K���������������Ŀ����______
��װ��D�еĻ�ѧ����ʽΪ______
��װ��B������CO2������Ϊ______g��ˮ����______��MgCO3��
A��һ�� B��һ���� C������ D����ȷ��
[��˼����]
���֤��ˮ����ȷʵ��������þ��С��������Լ����뷨��
��ȡ����Ϊ3g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����Wg���������ʵ����ۣ��ж�W��ֵ����______����ʱ��ˮ����һ������Mg��OH��2��