��Ŀ����
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷֽ���������̽������������»�����ش�������⣮
���������ϡ�ˮ������Ҫ�ɷ���Mg��OH��2��CaCO3 ��MgCO3�е�һ�ֻ����
����Ʒ�����
ʵ��1��ȷ��ˮ�����Ƿ�MgCO3
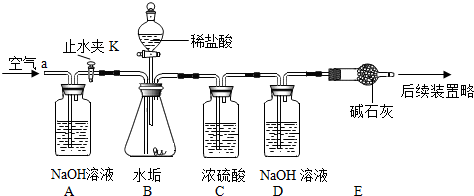
��������ʵ��װ�ã����ʵ��̽��������Ҫʵ�鲽�跢�£�

����D��Eװ��������Ϊ600.00g������ͼ��װ��2.50gˮ������������ƿ�У���������ϡ������Һ������ƿ�в��ٲ�������ʱ����ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ601.25g ����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
��ʵ�����ۡ�
��1��װ��B����������Ļ�ѧ����ʽΪ
��2��װ��B������CO2������Ϊ
��3��ˮ�����Ƿ�MgCO3��
С���ͬѧ��������Ϊ�ⶨһ��������CaCO3���������ᷴӦ���ɵĶ�����̼�������Ϳ���ȷ��������ͨ������˵��ˮ�����Ƿ�MgCO3��
ʵ��2��ȷ��ˮ�����Ƿ���Mg��OH��2��
��4����ȡ����Ϊ2.50g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����w g�����������ʵ����ۣ��ж�w��ֵ����
���������ϡ�ˮ������Ҫ�ɷ���Mg��OH��2��CaCO3 ��MgCO3�е�һ�ֻ����
����Ʒ�����
ʵ��1��ȷ��ˮ�����Ƿ�MgCO3
��������ʵ��װ�ã����ʵ��̽��������Ҫʵ�鲽�跢�£�

����D��Eװ��������Ϊ600.00g������ͼ��װ��2.50gˮ������������ƿ�У���������ϡ������Һ������ƿ�в��ٲ�������ʱ����ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ601.25g ����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
��ʵ�����ۡ�
��1��װ��B����������Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��ֻҪ��дһ�֣��������������������װ���ڵĶ�����̼�ų�����D��E�������
��װ���ڵĶ�����̼�ų�����D��E�������
����2��װ��B������CO2������Ϊ
1.25
1.25
g����3��ˮ�����Ƿ�MgCO3��
С���ͬѧ��������Ϊ�ⶨһ��������CaCO3���������ᷴӦ���ɵĶ�����̼�������Ϳ���ȷ��������ͨ������˵��ˮ�����Ƿ�MgCO3��
ʵ��2��ȷ��ˮ�����Ƿ���Mg��OH��2��
��4����ȡ����Ϊ2.50g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����w g�����������ʵ����ۣ��ж�w��ֵ����
w��56.8
w��56.8
����ʱ��ˮ����һ������Mg��OH��2����������1������̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ��������̼��дװ��B����������Ļ�ѧ����ʽ����Ӧ��ͨ�������Ϊ�˰ѷ�Ӧ���ɵĶ�����̼�ŵ�D�У��Ӷ���������գ�
��2������װ��D�������ж����ɵĶ�����̼������Ϊ1.25g��
��ˮ����Ϊ2.50g̼��ƣ����ݷ���ʽ��������ɵĶ�����̼1.1g�����Ժ�̼��þ��
����̼��ơ�̼��þ��������þ�����ᷴӦ�ķ���ʽ���ֱ��������2.50g�������������������������þ������࣬���Ϊ̼��þ������2.50gȫΪ̼��þ����������56.8g��w��56.8gʱ������������þ��
��2������װ��D�������ж����ɵĶ�����̼������Ϊ1.25g��
��ˮ����Ϊ2.50g̼��ƣ����ݷ���ʽ��������ɵĶ�����̼1.1g�����Ժ�̼��þ��
����̼��ơ�̼��þ��������þ�����ᷴӦ�ķ���ʽ���ֱ��������2.50g�������������������������þ������࣬���Ϊ̼��þ������2.50gȫΪ̼��þ����������56.8g��w��56.8gʱ������������þ��
����⣺��1��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ��������̼����ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2������Ӧ�������ֹˮ��K���������������Ϊ�˽�װ���еĶ�����̼ȫ�����գ�
��2��װ��D��E������������Һ����ʯ�ҿ��������̼��ַ�Ӧ�����ص�������Ϊ������̼���������ǣ�601.25g-600.00g=1.25g��
��3����ˮ����Ϊ2.50g̼��ƣ����ɶ�����̼����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
2.5g x
=
x=1.1g��С��1.25g����һ�����������ɶ�����̼��̼��þ���ڣ�
��4����ʵ��2��֪��2.5gˮ�������ᷴӦ����1.25g������̼����̼��ơ�̼��þ�����ᷴӦ����ʽ��֪����1.25g������̼ǡ����Ҫ3.65%��ϡ�����������
����Ҫϡ�����������x����CaCO3+2HCl=CaCl2+H2O+CO2����MgCO3+2HCl=MgCl2+H2O+CO2����֪��
2HCl��CO2
73 44
3.65%x 1.25g
=
x=56.8g
��CaCO3+2HCl=CaCl2+H2O+CO2����MgCO3+2HCl=MgCl2+H2O+CO2����Mg��OH��2+2HCl=MgCl2+2H2O��֪��
CaCO3��2HCl��MgCO3��Mg��OH��2��
100 73 84 58
��֪����������̼��þ��̼��ơ�������þ��������þ��������������࣬���Ե�ʵ������������������56.8ʱ��ˮ����һ������������þ��
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2������װ���ڵĶ�����̼�ų�����Dװ�����գ�
��2��1.25��
��3����ˮ����Ϊ2.50g̼��ƣ����ɶ�����̼����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
2.5g x
=
x=1.1g��С��1.25g����һ�����������ɶ�����̼��̼��þ���ڣ�
��4��w��56.8��
��2��װ��D��E������������Һ����ʯ�ҿ��������̼��ַ�Ӧ�����ص�������Ϊ������̼���������ǣ�601.25g-600.00g=1.25g��
��3����ˮ����Ϊ2.50g̼��ƣ����ɶ�����̼����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
2.5g x
| 100 |
| 2.5g |
| 44 |
| x |
x=1.1g��С��1.25g����һ�����������ɶ�����̼��̼��þ���ڣ�
��4����ʵ��2��֪��2.5gˮ�������ᷴӦ����1.25g������̼����̼��ơ�̼��þ�����ᷴӦ����ʽ��֪����1.25g������̼ǡ����Ҫ3.65%��ϡ�����������
����Ҫϡ�����������x����CaCO3+2HCl=CaCl2+H2O+CO2����MgCO3+2HCl=MgCl2+H2O+CO2����֪��
2HCl��CO2
73 44
3.65%x 1.25g
| 73 |
| 3.65%x |
| 44 |
| 1.25g |
x=56.8g
��CaCO3+2HCl=CaCl2+H2O+CO2����MgCO3+2HCl=MgCl2+H2O+CO2����Mg��OH��2+2HCl=MgCl2+2H2O��֪��
CaCO3��2HCl��MgCO3��Mg��OH��2��
100 73 84 58
��֪����������̼��þ��̼��ơ�������þ��������þ��������������࣬���Ե�ʵ������������������56.8ʱ��ˮ����һ������������þ��
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2������װ���ڵĶ�����̼�ų�����Dװ�����գ�
��2��1.25��
��3����ˮ����Ϊ2.50g̼��ƣ����ɶ�����̼����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
2.5g x
| 100 |
| 2.5g |
| 44 |
| x |
x=1.1g��С��1.25g����һ�����������ɶ�����̼��̼��þ���ڣ�
��4��w��56.8��
���������⿼���˳������ʳɷֵ��ƶϣ���ɴ��⣬�����������ʵ����ʽ������ṩ����Ϣ���У��ѶȽϴ��漰�ļ���϶࣬Ҫ������֪ʶϸ�ķ�������ܺܺÿ���ѧ����������������������

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷֽ���������̽������������»�����ش�������⣮
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3_�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������--ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
[�������]ˮ������Ҫ�ɷ���һ������ ���ѧʽ����ͬ���� �����ܺ���Ca��OH��2��MgCO3��
[��Ʒ���]ʵ��1��ȷ��ˮ�����Ƿ�Ca��OH��2
ʵ��2��ȷ��ˮ�����Ƿ�MgCO3
��2.50gˮ����Ʒ������ƿ�У�����������ϡ���ᣬ�������ɵĶ�����̼����1.25g��
��ش�
��1��д��̼��������ᷴӦ�Ļ�ѧ����ʽ ��
��2����ʽ���㣬���ˮ����û��̼��þ����̼��Ƶ�����Ϊ���٣�
��3��ˮ���� ��MgCO3������ĸ����
A��һ�� B��һ���� C������ D����ȷ����
�����ǣ� ��
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3_�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������--ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
| ���ָơ�þ�������ܽ��Ա���20�棩 | |||
| OH- | CO32- | HCO3- | |
| Ca2+ | �� | ���� | ���� |
| Mg2+ | ���� | �� | ���� |
[��Ʒ���]ʵ��1��ȷ��ˮ�����Ƿ�Ca��OH��2
| ʵ�鲽�� | ʵ������ | �йػ�ѧ��Ӧ����ʽ |
| �����������ˮ���м�������������ˮ��ֽ��裬���ˣ�����Һ�м���Na2CO3��Һ�� | �а�ɫ�������� |
��2.50gˮ����Ʒ������ƿ�У�����������ϡ���ᣬ�������ɵĶ�����̼����1.25g��
��ش�
��1��д��̼��������ᷴӦ�Ļ�ѧ����ʽ
��2����ʽ���㣬���ˮ����û��̼��þ����̼��Ƶ�����Ϊ���٣�
��3��ˮ����
A��һ�� B��һ���� C������ D����ȷ����
�����ǣ�
ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷݽ���������̽������������»�����ش�������⣮
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������----ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
���ָơ�þ�������ܽ��Ա���20�棩
[�������]
����һ��ˮ���ijɷ���______
�������ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2
��������ˮ���ijɷ���CaCO3��Mg��OH��2��MgCO3
�����ģ�ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2 MgCO3
[�������]
��1��ȷ��ˮ�����Ƿ����������ƣ�2��ȷ��ˮ�����Ƿ���̼��þ
[ʵ�鲽��]
��1��
��2��������ͼʵ��װ����ɣ�2����̽��������Ҫʵ�鲽�����£�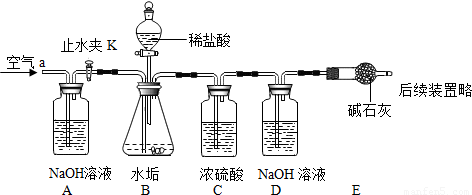
����D��Eװ��������Ϊ400.00g
����ͼ��װ��3gˮ����Ʒ������ƿ�У���������ϡ��������ƿ�в��ٲ������ݣ��رշ�Һ©������
��ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ401.54g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
����ʵ��ش��������⣺
�ټ�����Ʒǰ��Ӧ______��װ��C��������______����Ӧ�������ֹˮ��K���������������Ŀ����______
��װ��D�еĻ�ѧ����ʽΪ______
��װ��B������CO2������Ϊ______g��ˮ����______��MgCO3��
A��һ�� B��һ���� C������ D����ȷ��
[��˼����]
���֤��ˮ����ȷʵ��������þ��С��������Լ����뷨��
��ȡ����Ϊ3g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����Wg���������ʵ����ۣ��ж�W��ֵ����______����ʱ��ˮ����һ������Mg��OH��2��
[��������]��Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С������----ˮ������Ҫ�ɷ�Ϊ̼���κͼ���й����ʵ��ܽ��Լ��±���
���ָơ�þ�������ܽ��Ա���20�棩
| ������ ������ | OH- | CO32- | HCO3- |
| Ca2+ | �� | ���� | ���� |
| Mg2+ | ���� | �� | ���� |
����һ��ˮ���ijɷ���______
�������ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2
��������ˮ���ijɷ���CaCO3��Mg��OH��2��MgCO3
�����ģ�ˮ���ijɷ���CaCO3��Mg��OH��2��Ca��0H��2 MgCO3
[�������]
��1��ȷ��ˮ�����Ƿ����������ƣ�2��ȷ��ˮ�����Ƿ���̼��þ
[ʵ�鲽��]
��1��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �����������ˮ���м�������������ˮ��ֽ��裬���ˣ�����Һ�м���______��Һ | ��______���� | ˮ������ �������� |

����D��Eװ��������Ϊ400.00g
����ͼ��װ��3gˮ����Ʒ������ƿ�У���������ϡ��������ƿ�в��ٲ������ݣ��رշ�Һ©������
��ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ401.54g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������װ���ڿ�����ʵ���Ӱ�죩��
����ʵ��ش��������⣺
�ټ�����Ʒǰ��Ӧ______��װ��C��������______����Ӧ�������ֹˮ��K���������������Ŀ����______
��װ��D�еĻ�ѧ����ʽΪ______
��װ��B������CO2������Ϊ______g��ˮ����______��MgCO3��
A��һ�� B��һ���� C������ D����ȷ��
[��˼����]
���֤��ˮ����ȷʵ��������þ��С��������Լ����뷨��
��ȡ����Ϊ3g��ˮ��������3.65%��ϡ������֮��Ӧ���������ϡ����Wg���������ʵ����ۣ��ж�W��ֵ����______����ʱ��ˮ����һ������Mg��OH��2��