��Ŀ����
ijУ��ѧʵ���ҷ�ҺͰ���ռ����ܽ����FeSO4��CuSO4�ķ�ˮ����ֱ���ŷŵ���ˮ����������ؽ�����Ⱦ��������˷ѣ�������2�����ͬѧ�������ø�ѧ���ġ������������˳�������������йػ�ѧ֪ʶ�Է�ˮ���д�����
��1����ϰ���������˳��������±��ո��зֱ������Ӧ��Ԫ�ط��ţ� _________ �� _________ �� _________ ��
��1����ϰ���������˳��������±��ո��зֱ������Ӧ��Ԫ�ط��ţ� _________ �� _________ �� _________ ��

��2����Ƴ�ȥͭ���ӣ������������������ͭ��ʵ�鷽���������й����⣺
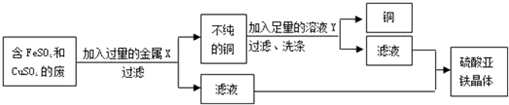
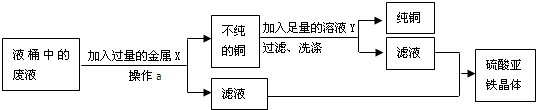
�ٷ�ˮ�ڼ������Xǰ����й��ˣ�Ŀ���ǽ����� _________ �����ʳ�ȥ�����й��˵IJ�������������У� _________ �� _________ �� _________ ��___________�������ͼ��ѡ�������������������������д����


�ڽ���X�� _________ ��������ˮ������Ӧ�Ļ�ѧ����ʽΪ _________ ����ҺY�� _________ ���������ȥͭ�����ʣ��йط�Ӧ�Ļ�ѧ����ʽΪ _________ ��
�۴���Һ�л�ȡ������������IJ��������� _________ ��
�۴���Һ�л�ȡ������������IJ��������� _________ ��
��1��Na��Zn��Ag��
��2���ٲ��ܣ�����̨��©�����ձ�����������
��Fe����������Fe+CuSO4=FeSO4+Cu��ϡ���Fe+H2SO4=FeSO4+H2����
�۽ᾧ���������ᾧ����ȴ�ᾧ����
��2���ٲ��ܣ�����̨��©�����ձ�����������
��Fe����������Fe+CuSO4=FeSO4+Cu��ϡ���Fe+H2SO4=FeSO4+H2����
�۽ᾧ���������ᾧ����ȴ�ᾧ����

��ϰ��ϵ�д�
�����Ŀ