��Ŀ����
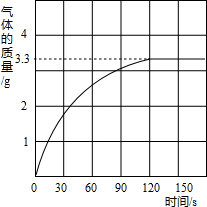 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ����1��ˮ����̼��Ƶ����������Ƕ��٣�
��2������ˮ���г�̼��ƺ�������þ�⣬�������������ʣ��ܽ�10gˮ����������Ҫ��������Ϊ10%�������������
��������1�����ݲ���CO2��������ͼ���ɵ�֪̼�����ȫ��Ӧʱ�ų�������̼3.3g���ɷų�������̼������������̼��������ᷴӦ�Ļ�ѧ����ʽ�������ˮ����Ʒ��̼��Ƶ��������Ӷ�����̼�����ˮ����Ʒ�������ȼ�ˮ����̼��Ƶ�����������
��2���ֱ����ˮ����̼�����������þ��ȫ��Ӧ������HCl��������������Һ�����������������㹫ʽ����������������Һ��������������������Һ��������
��2���ֱ����ˮ����̼�����������þ��ȫ��Ӧ������HCl��������������Һ�����������������㹫ʽ����������������Һ��������������������Һ��������
����⣺��1�����ݲ���CO2��������ͼ���ɵ�֪̼�����ȫ��Ӧʱ�ų�������̼3.3g����ˮ����̼��Ƶ�����Ϊx������HCl������Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
x y 3.3g
=
x=7.5g
=
y=5.475g
ˮ����̼��Ƶ���������=
��100%=75%
��ˮ����̼��Ƶ�����������75%��
��2����������þ��ȫ��Ӧ����HCl������Ϊz
2HCl+Mg��OH��2�TMgCl2+2H2O
73 58
z 10g-7.5g=2.5g
=
z��3.147g
������Ҫ��������Ϊ10%�����������=
=86.2g
�ʴ�Ϊ��86.2g��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
x y 3.3g
| 100 |
| x |
| 44 |
| 3.3g |
| 73 |
| y |
| 44 |
| 3.3g |
ˮ����̼��Ƶ���������=
| 7.5g |
| 10g |
��ˮ����̼��Ƶ�����������75%��
��2����������þ��ȫ��Ӧ����HCl������Ϊz
2HCl+Mg��OH��2�TMgCl2+2H2O
73 58
z 10g-7.5g=2.5g
| 73 |
| z |
| 58 |
| 2.5g |
������Ҫ��������Ϊ10%�����������=
| 5.475g+3.147g |
| 10% |
�ʴ�Ϊ��86.2g��
��������ʾ�仯�����ߵ��۵��������ʱˮ����̼�����ȫ��Ӧ�ų�������̼�����ֵ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��  ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��
ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ�� ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��