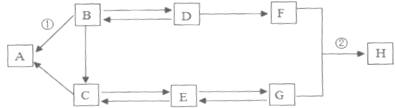
��Ŀ����
����Ŀ����������ͬ���������ķ�չ�����Ľ�����ϵʮ�����С�
ʵ�����ù�ҵ���죨��Ҫ�ɷ���![]() ��������������
��������������![]() ��
��![]() ��ģ�ҵ�������ⶨ��������Ԫ�ص�����������������ʵ�飬װ�����£�
��ģ�ҵ�������ⶨ��������Ԫ�ص�����������������ʵ�飬װ�����£�
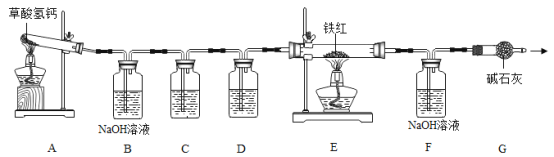
�����ϣ�
��������ǰ�ɫ���壬��ѧʽΪ![]() �������ֽ⣬�������������
�������ֽ⣬�������������
��![]() ��Һ�ܺܺõ�����
��Һ�ܺܺõ�����![]() ����ʯ��������
����ʯ��������![]() ��
��![]() ��
��
��Ũ���������ˮ�ԣ���Һ��������
���������ۣ�
��1��ʵ��ǰӦ��_____________________��
��2��д��A�з����ķ�Ӧ��ѧ����ʽ��_____________________��
��3������E�е������Ǵ����������![]() ����C��D�е��Լ�������_____��_____������ĸ����
����C��D�е��Լ�������_____��_____������ĸ����
a Ũ���� b �����ʯ��ˮ c ����������Һ
��4��Cװ�õ�������______________________��
��5��ʵ�������Eװ��ֹͣ���ȣ�����ͨ��![]() ����ȴ����Ŀ����______��
����ȴ����Ŀ����______��
��6��д��Eװ����![]() �������ķ�Ӧ��ѧ����ʽ��_________________��
�������ķ�Ӧ��ѧ����ʽ��_________________��
��7����ȡ������Ʒ7.0g��������װ�ý���ʵ�飬�ⶨ��������Ԫ�ص�����������ʵ��ǰ��Ƶ�Fװ������4.4g�������������Ԫ�ص�����������________��
��ʵ�鷴˼��
��8����ʵ�������ȱ��Gװ�ã��������������أ���������Ʒ����������������________������ƫ������������������ƫС������
��9����ָ������ʵ��װ�õ�һ������ȱ��___________��
���𰸡����װ�������� Ca(HC2O4)2![]() CaO+H2O+2CO2��+2CO�� b a ���������̼�Ƿ�Bװ����ȫ���� ��ֹFװ���е�Һ�嵹��ը�Ѳ�����
CaO+H2O+2CO2��+2CO�� b a ���������̼�Ƿ�Bװ����ȫ���� ��ֹFװ���е�Һ�嵹��ը�Ѳ����� 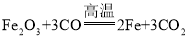 77.1% ƫС û�д���β��
77.1% ƫС û�д���β��
��������
��1��ʵ��ǰӦ�ȼ��װ�������ԡ�
��2�������Ͽ�֪��������Ƽ����ֽ⣬�����������������A�з����ķ�Ӧ��ѧ����ʽΪ��Ca(HC2O4)2![]() CaO+H2O+2CO2��+2CO����
CaO+H2O+2CO2��+2CO����
��3��������̼�ܺ��������Ʒ�Ӧ����̼��ƺ�ˮ��Ũ�����ܹ�����ˮ���������Խ���E�е������Ǵ����������CO����C��D�е��Լ������dz���ʯ��ˮ��Ũ���ᡣ
���b��a��
��4��������̼��ʹ����ʯ��ˮ����ǣ�Cװ�õ������Ǽ��������̼�Ƿ�Bװ����ȫ���ա�
��5��ʵ�������Eװ��ֹͣ���ȣ�����ͨ��CO����ȴ����Ŀ���Ƿ�ֹFװ���е�Һ�嵹��ը�Ѳ����ܡ�
��6��Eװ����Fe2O3��һ����̼�����ķ�Ӧ��ѧ����ʽΪ��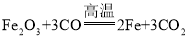 ��
��
��7����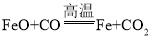 ��
��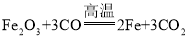 ��
��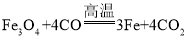 ����֪��������̼�е���Ԫ��һ���������������������Ʒ����Ԫ������Ϊ��
����֪��������̼�е���Ԫ��һ���������������������Ʒ����Ԫ������Ϊ��![]() �������������Ԫ�ص����������ǣ�
�������������Ԫ�ص����������ǣ�![]() ��
��
��8����ʵ�������ȱ��Gװ�ã��������������أ��������еĶ�����̼�ᱻ����������Һ���գ��ᵼ�¶�����̼����ƫ�Ӷ�������Ʒ����Ԫ������ƫ��һ��������Ʒ����Ԫ������ƫС��������Ʒ����������������ƫС��
��9������ʵ��װ�õ�һ������ȱ����û�д���β����

 ͬ����ϰǿ����չϵ�д�
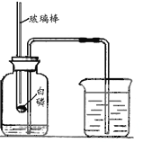
ͬ����ϰǿ����չϵ�д�����Ŀ��ijͬѧ����ʯ�ҽ���ˢǽ�ڣ������ǽ��ͱ�Ӳ����������չ������̽����
��ͬѧȡС����Ʒ��һ֧�Թ��У�����һ����������ˮ���������ˣ��õ���ɫ��������Һ��
��1����ͬѧ�ⶨ����Һ�����ȣ�����Ϊ���������________________________��
��2����ͬѧ�ֶ�ɫ�����������������¼������
����1 ֻ��Ca��OH��2
����2 ֻ��![]()
����3 ________________
��3��Ϊ���ж��⼸�ּ���ĺ����ԣ���ͬѧ��������ʵ�飬������������ʵ�鱨�档
ʵ����� | ʵ������ | ʵ����ۣ��û�ѧ����ʽ��ʾ�� |
________________ | ________________ | _________________________����1�������� |
��4��Ϊ�˵õ������ۣ�����ȡ��������������һ֧�Թ��У�������ˮ�����ú�����ɫ��̪��Һ����Һ�Ժ�ɫ��˵��ֻ�м���________������
����Ŀ����þ�Ͻ����ܶ�С��ǿ�ȸߡ����Ժõ��ص���ص㣬������������Ӧ�ù㷺��ij��ȤС���ͬѧ�Ա��Ϊ�١��ڵ�������þ�Ͻ���Ʒ����������ʵ�顣
��֪��þ�����ᷴӦ�������������Һ��Ӧ�����������ᷴӦ���������������Ƶȼ���Һ��Ӧ����ѧ����ʽΪ��![]() ��
��![]() ��Һ�������̼��Ӧ�ܲ��������������������߾����ˡ�ϴ�ӡ����պ�������Ӧ�����
��Һ�������̼��Ӧ�ܲ��������������������߾����ˡ�ϴ�ӡ����պ�������Ӧ�����
��1��ȡ����Ϊ![]() ����Ʒ�ٺ���������������Һ��Ӧ��Ȼ����ˣ�������Һ��ͨ������Ķ�����̼���壬�����ó������ˡ�ϴ�ӡ���ɡ����գ����ù����������Ϊ
����Ʒ�ٺ���������������Һ��Ӧ��Ȼ����ˣ�������Һ��ͨ������Ķ�����̼���壬�����ó������ˡ�ϴ�ӡ���ɡ����գ����ù����������Ϊ![]() ������Ʒ������������������________
������Ʒ������������������________
��2��ȡ��ͬ��������Ʒ�ڷֱ��30g��ͬŨ�ȵ����ᷴӦ����ȡ�Ͻ�������������������������ܶ�Ϊ0.0893g/L�����±���ʾ��
ʵ����� | a | b | c |
�Ͻ�����mg | 510 | 765 | 918 |
�������mL | 560 | 672 | 672 |
�Ͻ�����þ�������ȡ�________