��Ŀ����
��ѧ��ȤС���������Ʒ������������������̽����
[��������]�����г����⣬������̼���衢�����Ԫ�أ�̼����������������ʽ���ڣ�һ���ǵ���̼��C������һ���ǻ���̼����Fe3C�����衢�ס��������ʽ��Ϊ���ӣ���Щ����ͨ��������ϡ���ᷢ����ѧ��Ӧ��
[ʵ������]
| �ձ� | �ձ�+������Ʒ | ϡ���� | �ձ�+ʣ������ | |
| ����/g | 70.4 | 102.4 | 247.3 | 348.7 |
��2�����ʼ첿�ž�ȷ�ⶨ������Ʒ����Ԫ�ص��ܺ���Ϊ95.2%������û�������ᷴӦ����������Fe3C��ʽ���ڣ�������Ʒ��Fe3C������������
������Ʒ������=102.4g-70.4g=32g
����Ʒ�е�����������Ϊx
Fe+H2SO4=FeSO4+H2��
56 2
x 1g
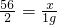 ��x=28g
��x=28g 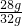 ��100%=87.5%
��100%=87.5%�𣺸�������Ʒ�е���������������Ϊ87.5%
��2��32g��95.2%-28g=2.464g
2.464g��
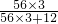 =2.64g
=2.64g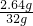 ��100%=8.25%
��100%=8.25%����Ʒ��Fe3C����������Ϊ8.25%��
��������1���ձ���ǰ����ٵ������������������������������ᷴӦ�Ļ�ѧ����ʽ����������������������������������ٸ���
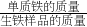 ��100%���������Ʒ�е�����������������
��100%���������Ʒ�е�������������������2����Ʒ����������Ʒ����Ԫ�ص���������=��Ʒ����Ԫ�ص���������������Ʒ����Ԫ�ص�������-���������������Fe3C����Ԫ�ص�������������������Ԫ�ص��������Fe3C��������
������������ĵڶ�С��Ƚ��ѣ�����������ʱӦ���������ֵ�����ǰ������ֱ��������֪������

[��������]�����г����⣬������̼���衢�����Ԫ�أ�̼����������������ʽ���ڣ�һ���ǵ���̼��C������һ���ǻ���̼����Fe3C�����衢�ס��������ʽ��Ϊ���ӣ���Щ����ͨ��������ϡ���ᷢ����ѧ��Ӧ��
[ʵ������]
| �ձ� | �ձ�+������Ʒ | ϡ���� | �ձ�+ʣ������ | |
| ����/g | 70.4 | 102.4 | 247.3 | 348.7 |
��2�����ʼ첿�ž�ȷ�ⶨ������Ʒ����Ԫ�ص��ܺ���Ϊ95.2%������û�������ᷴӦ����������Fe3C��ʽ���ڣ�������Ʒ��Fe3C������������
(3��) ��ѧ��ȤС���������Ʒ������������������̽����
���������ϡ������г����⣬������̼���衢�����Ԫ�ء�̼����������������ʽ���ڣ�һ���ǵ���̼��C������һ���ǻ���̼(��Fe3C)���衢�ס��������ʽ��Ϊ���ӣ���Щ����ͨ��������ϡ���ᷢ����ѧ��Ӧ��
��ʵ�����ݡ�
|
|
�ձ� |
�ձ�+������Ʒ |
ϡ���� |
�ձ�+ʣ������ |
|
����/g |
70.4 |
102.4 |
247.3 |
348.7 |
����㣺(1)��������Ʒ�е�����������������
(2)���ʼ첿�ž�ȷ�ⶨ������Ʒ����Ԫ�ص��ܺ���Ϊ95.2%(����û�������ᷴӦ����������Fe3C��ʽ����)������Ʒ��Fe3C������������
��ѧ��ȤС���������Ʒ������������������̽����
���������ϡ������г����⣬������̼���衢�����Ԫ�ء�̼����������������ʽ���ڣ�һ���ǵ���̼��C������һ���ǻ���̼(��Fe3C)���衢�ס��������ʽ��Ϊ���ӣ���Щ����ͨ��������ϡ���ᷢ����ѧ��Ӧ��
��ʵ�����ݡ�
| �ձ� | �ձ�+������Ʒ | ϡ���� | �ձ�+ʣ������ | |
| ����/g | 70.4 | 102.4 | 247.3 | 348.7 |
����㣺(1)��������Ʒ�е�����������������
(2)���ʼ첿�ž�ȷ�ⶨ������Ʒ����Ԫ�ص��ܺ���Ϊ95.2%(����û�������ᷴӦ����������Fe3C��ʽ����)������Ʒ��Fe3C������������
[��������]�����г����⣬������̼���衢�����Ԫ�أ�̼����������������ʽ���ڣ�һ���ǵ���̼��C������һ���ǻ���̼����Fe3C�����衢�ס��������ʽ��Ϊ���ӣ���Щ����ͨ��������ϡ���ᷢ����ѧ��Ӧ��
[ʵ������]
| �ձ� | �ձ�+������Ʒ | ϡ���� | �ձ�+ʣ������ | |
| ����/g | 70.4 | 102.4 | 247.3 | 348.7 |
��2�����ʼ첿�ž�ȷ�ⶨ������Ʒ����Ԫ�ص��ܺ���Ϊ95.2%������û�������ᷴӦ����������Fe3C��ʽ���ڣ�������Ʒ��Fe3C������������