ΧβΡΩΡΎ»ί
Ρ≥ΜνΕ·–ΓΉιΗυΨίœ¬ΆΦΥυ ΨΫχ––ΡΘΡβΝΕΧζΒΡ Β―ιΘ§≤ΔΕ‘≤ζΈοΒΡ≥…Ζ÷Ϋχ––ΧΫΨΩΓΘ
ΓΨΧα≥ωΈ ΧβΓΩΚΎ…ΪΖέΡ©AΩ…Ρή « ≤Ο¥ΡΊΘΩ
ΓΨ≤ι‘ΡΉ ΝœΓΩ–ΓΜΣ≤ι‘ΡΉ ΝœΘ§ΒΟΒΫΙΊ”ΎΧζΒΡ―θΜ·Έο»γœ¬–≈œΔΘΚ
ΓΨΧα≥ω≤¬œκΓΩΘ®1Θ©–ΓΟς»œΈΣΚΎ…ΪΖέΡ©A»Ϊ≤Ω «ΧζΖέΓΘΈΣΝΥ÷ΛΟςΉ‘ΦΚΒΡ≤¬œκ–ΓΟς…ηΦΤΝΥ»γ…œΆΦΒΡ Β―ιΘ§ΗΟ Β―ι÷–Θ§–ΓΟςΦ”»κΒΡX»ή“Κ « ΘΜ Β―ιΙΐ≥Χ÷–≤ΌΉςΔώ « ’βΗω Β―ιΜυ±Ψ≤ΌΉςΓΘ
–ΓΜΣΗυΨί–ΓΟςΒΡ Β―ι»œΕ®–ΓΟςΒΡ≤¬œκ «¥μΈσΒΡΘ§άμ”… « ΓΘ
Θ®2Θ©–ΓΜΣΗυΨίΉ ΝœΧα≥ωΝΥΉ‘ΦΚΒΡΝΫΗω≤¬œκΘ§«κΫαΚœΥυ―ß÷Σ Ε≤Ι≥δ”ύœ¬ΒΡ“ΜΗω≤¬œκΓΘ
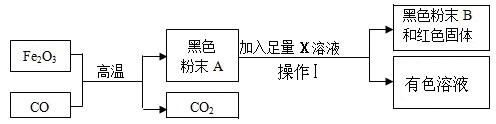
≤¬œκΔΌΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©Ήι≥…
≤¬œκΔΎΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆ―θΜ·―«ΧζΘ®FeOΘ©Ήι≥…
≤¬œκΔέΘΚ
ΓΨ Β―ιΧΫΨΩΓΩ
ΓΨΖ¥ΥΦΤάΦέΓΩ–ΓΨξ»œΈΣΖΫΑΗΔΎ÷–ΒΟ≥ωΒΡΫα¬έ≤Μ’ΐ»ΖΘ§Τδάμ”… « ΓΘ
ΓΨΒΟ≥ωΫα¬έΓΩΚΎ…ΪΖέΡ©A «ΧζΚΆ―θΜ·―«ΧζΒΡΜλΚœΈοΓΘ
–ΓΜΣœκ≤βΕ®ΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐΘ§…ηΦΤΝΥ»γœ¬ Β―ιΘΚ
»Γ8gΚΎ…ΪΖέΡ©AΦ”»κ50gΉψΝΩΒΡœΓΝρΥαΘ§≥δΖ÷Ζ¥”ΠΚσ≥ΤΝΩΘ§ Θ”ύΈο÷ ΒΡ÷ ΝΩΈΣ57.8gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «Εύ…ΌΘΩΘ®–¥≥ωΦΤΥψΙΐ≥ΧΘ©

ΓΨΧα≥ωΈ ΧβΓΩΚΎ…ΪΖέΡ©AΩ…Ρή « ≤Ο¥ΡΊΘΩ
ΓΨ≤ι‘ΡΉ ΝœΓΩ–ΓΜΣ≤ι‘ΡΉ ΝœΘ§ΒΟΒΫΙΊ”ΎΧζΒΡ―θΜ·Έο»γœ¬–≈œΔΘΚ
| ΧζΒΡ―θΜ·Έο | Fe2O3 | Fe3O4 | FeO |
| ―’…Ϊ | Κλ | ΚΎ | ΚΎ |
| Έοάμ–‘÷ | ≤ΜΡή±Μ¥≈ΧζΈϋ“ΐ | Ρή±Μ¥≈ΧζΈϋ“ΐ | ≤ΜΡή±Μ¥≈ΧζΈϋ“ΐ |
| Μ·―ß–‘÷ | Ω…»ή”ΎΥα | ≥ΘΈ¬œ¬≤Μ»ή”ΎœΓΥα | Ω…»ή”ΎΥαΘ§ΒΪ≤Μ…ζ≥…ΤχΧε |
ΓΨΧα≥ω≤¬œκΓΩΘ®1Θ©–ΓΟς»œΈΣΚΎ…ΪΖέΡ©A»Ϊ≤Ω «ΧζΖέΓΘΈΣΝΥ÷ΛΟςΉ‘ΦΚΒΡ≤¬œκ–ΓΟς…ηΦΤΝΥ»γ…œΆΦΒΡ Β―ιΘ§ΗΟ Β―ι÷–Θ§–ΓΟςΦ”»κΒΡX»ή“Κ « ΘΜ Β―ιΙΐ≥Χ÷–≤ΌΉςΔώ « ’βΗω Β―ιΜυ±Ψ≤ΌΉςΓΘ
–ΓΜΣΗυΨί–ΓΟςΒΡ Β―ι»œΕ®–ΓΟςΒΡ≤¬œκ «¥μΈσΒΡΘ§άμ”… « ΓΘ
Θ®2Θ©–ΓΜΣΗυΨίΉ ΝœΧα≥ωΝΥΉ‘ΦΚΒΡΝΫΗω≤¬œκΘ§«κΫαΚœΥυ―ß÷Σ Ε≤Ι≥δ”ύœ¬ΒΡ“ΜΗω≤¬œκΓΘ
≤¬œκΔΌΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©Ήι≥…
≤¬œκΔΎΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆ―θΜ·―«ΧζΘ®FeOΘ©Ήι≥…
≤¬œκΔέΘΚ
ΓΨ Β―ιΧΫΨΩΓΩ
| ΖΫΑΗ | Β―ι≤ΌΉς | Ω…ΡήΒΡœ÷œσ | Ϋα¬έ |
| ΔΌ | »Γ ΝΩΚΎ…ΪΖέΡ©A ”Ο¥≈ΧζΈϋ“ΐ | | ≤¬œκΔΌ’ΐ»Ζ |
| ΔΎ | ΝΩΚΎ…ΪΖέΡ©A ”Ο¥≈ΧζΈϋ“ΐ | ΚΎ…ΪΖέΡ©≤ΩΖ÷±ΜΈϋ“ΐ | ≤¬œκΔΎ’ΐ»Ζ |
| Δέ | | | ≤¬œκΔέ’ΐ»Ζ |
ΓΨΖ¥ΥΦΤάΦέΓΩ–ΓΨξ»œΈΣΖΫΑΗΔΎ÷–ΒΟ≥ωΒΡΫα¬έ≤Μ’ΐ»ΖΘ§Τδάμ”… « ΓΘ
ΓΨΒΟ≥ωΫα¬έΓΩΚΎ…ΪΖέΡ©A «ΧζΚΆ―θΜ·―«ΧζΒΡΜλΚœΈοΓΘ
–ΓΜΣœκ≤βΕ®ΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐΘ§…ηΦΤΝΥ»γœ¬ Β―ιΘΚ
»Γ8gΚΎ…ΪΖέΡ©AΦ”»κ50gΉψΝΩΒΡœΓΝρΥαΘ§≥δΖ÷Ζ¥”ΠΚσ≥ΤΝΩΘ§ Θ”ύΈο÷ ΒΡ÷ ΝΩΈΣ57.8gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «Εύ…ΌΘΩΘ®–¥≥ωΦΤΥψΙΐ≥ΧΘ©
Θ®1Θ©ΝρΥαΆ≠»ή“ΚΓΔΙΐ¬ΥΘΜΦ”»κΉψΝΩΝρΥαΆ≠»ή“ΚΖ¥”ΠΚσΙΐ¬ΥΘ§ΜΙ”–ΚΎ…ΪΖέΡ©¥φ‘Ύ
Θ®2Θ©≤¬œκ3ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…
ΓΨΖ¥ΥΦΤάΦέΓΩΘΚΚ§”–ΥΡ―θΜ·»ΐΧζ ±Θ§“≤ΖΔ…ζΆ§―υΒΡœ÷œσ
ΦΤΥψΘΚ¬‘Θ§Υψ≥ωΧζΒΡ÷ ΝΩ1Ζ÷Θ§Υψ≥ω÷ ΝΩΖ÷ ΐ1Ζ÷
Θ®2Θ©≤¬œκ3ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…
| ΖΫΑΗ | Β―ι≤ΌΉς | Ω…ΡήΒΡœ÷œσ | Ϋα¬έ |
| ΔΌ | | ΚΎ…ΪΖέΡ©A»Ϊ≤Ω±ΜΈϋ“ΐ | |
| ΔΎ | | | |
| Δέ | »Γ ΝΩΚΎ…ΪΖέΡ©BΘ§Φ”»κΉψΝΩœΓ―ΈΥα Θ®Μρ’Ώ»Γ ΝΩΚΎ…ΪΖέΡ©B”Ο¥≈ΧζΈϋ“ΐΘΜΚΎ…ΪΖέΡ©≤ΩΖ÷±ΜΈϋ“ΐΘ© | ΚΎ…ΪΖέΡ©B≤ΩΖ÷»ήΫβ Θ®ΚΎ…ΪΖέΡ©B≤ΩΖ÷±ΜΈϋ“ΐΘ© | |
ΓΨΖ¥ΥΦΤάΦέΓΩΘΚΚ§”–ΥΡ―θΜ·»ΐΧζ ±Θ§“≤ΖΔ…ζΆ§―υΒΡœ÷œσ
ΦΤΥψΘΚ¬‘Θ§Υψ≥ωΧζΒΡ÷ ΝΩ1Ζ÷Θ§Υψ≥ω÷ ΝΩΖ÷ ΐ1Ζ÷
ΘΚΘ®1Θ©”…ΚΎ…ΪΖέΡ©AΦ”»κΉψΝΩX»ή“ΚΒΟΒΫ”–…Ϊ»ή“ΚΚΆΚΎ…ΪΙΧΧεΓΔΚλ…ΪΙΧΧεΘ§≥ΘΦϊΒΡΚλ…ΪΙΧΧε «Ά≠Θ§ΗυΨί÷ΟΜΜΖ¥”ΠΩ…÷ΣΧζΑ―Ά≠÷ΟΜΜ≥ωά¥ΝΥΘ§Υυ“‘Φ”»κΒΡ»ή“Κ «Ά≠ΒΡ―Έ»ή“ΚΘ§Ω…»œΈΣ «ΝρΥαΆ≠»ή“ΚΘ§ΝρΥαΆ≠”κΧζΖ¥”ΠΡή…ζ≥…Ά≠ΚΆΝρΥα―«ΧζΘ§ΝρΥα―«Χζ ««≥¬Χ…ΪΒΡΘΜΑ―≤Μ»ή”Ύ“ΚΧεΒΡΙΧΧε”κ“ΚΧεΖ÷άκΒΡΖΫΖ® «Ιΐ¬ΥΘΜ”…”ΎΦ”»κΒΡΝρΥαΆ≠ «ΉψΝΩΒΡΘ§Υυ“‘ΙΧΧε÷–ΒΡΧζΕΦΡή±ΜΖ¥”ΠΒτΘ§Ά®ΙΐΦλ≤βΖΔœ÷Φ”»κΝρΥαΆ≠ΚσΘ§ΜΙ”–ΚΎ…ΪΙΧΧε≤Μ»ήΈοΘ§ΥΒΟς≤ΜΩ…Ρή»Ϊ≤ΩΈΣΧζΘ§–ΓΟς»œΈΣΚΎ…ΪΖέΡ©A»Ϊ≤Ω «ΧζΖέΘ§Υυ“‘ΗΟΥΒΖ®¥μΈσΘΜ
Θ®2Θ©ΚΎ…ΪΖέΡ©”–ΘΚΧζΖέΓΔΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©ΓΔ―θΜ·―«ΧζΘ§Υυ“‘’β»ΐ÷÷œύΜΞΉιΚœ«ιΩω”–ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©Ήι≥…ΓΔΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆ―θΜ·―«ΧζΘ®FeOΘ©Ήι≥…ΓΔΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…ΘΜ“ρΈΣΧζΖέΡή±Μ¥≈ΧζΈϋ“ΐΘ§ΥΡ―θΜ·»ΐΧζ“≤Ρή±Μ¥≈ΧζΈϋ“ΐΘ§Υυ“‘»γΙϊ «’βΝΫ÷÷Έο÷ Ήι≥…Θ§œ÷œσΈΣΘΚΚΎ…ΪΖέΡ©A»Ϊ≤Ω±ΜΈϋ“ΐΘΜ”…”Ύ―θΜ·―«Χζ»ή”ΎΥαΘ§ΥΡ―θΜ·»ΐΧζ≤Μ»ή”ΎΥαΘ§Υυ“‘Φ”»κΥαΘ§Ω¥ΚΎ…ΪΙΧΧε «Ζώ±Μ»ήΫβΘ§»γΙϊ≤ΩΖ÷»ήΫβΘ§ΥΒΟςΚ§”–ΥΡ―θΜ·»ΐΧζΘΜ–ΓΨξ»œΈΣΖΫΑΗΔΎ÷–ΒΟ≥ωΒΡΫα¬έ≤Μ’ΐ»ΖΥΐ»œΈΣΚ§”–ΥΡ―θΜ·»ΐΧζ ±Θ§“≤ΖΔ…ζΆ§―υΒΡœ÷œσΘΜ
ΗυΨί÷ ΝΩΒΡΦθ…ΌΝΩ «…ζ≥…«βΤχΒΡ÷ ΝΩΘ§Υυ“‘…ζ≥…«βΤχΒΡ÷ ΝΩΈΣΘΚ8g+50g-57.8g=0.2gΘ§…η“Σ…ζ≥…0.2g«βΤχΘ§–η“ΣΧζ÷ ΝΩΈΣX‘ρΘΚFe+H2SO4=FeSO4+H2Γϋ
56 2
X 0.2g
ΗυΨίΘΚ ΫβΒΟX=5.6gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «
ΫβΒΟX=5.6gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «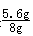 ΓΝ100%=70%Θ°
ΓΝ100%=70%Θ°
Ι ¥πΑΗΈΣΘΚΘ®1Θ©ΝρΥαΆ≠»ή“ΚΓΔΙΐ¬ΥΘΜΦ”»κΉψΝΩΝρΥαΆ≠»ή“ΚΖ¥”ΠΚσΙΐ¬ΥΘ§ΜΙ”–ΚΎ…ΪΖέΡ©¥φ‘Ύ
Θ®2Θ©≤¬œκ3ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…
Ζ¥ΥΦΤάΦέΘΚΚ§”–ΥΡ―θΜ·»ΐΧζ ±Θ§“≤ΖΔ…ζΆ§―υΒΡœ÷œσ
ΦΤΥψΘΚ70%
Θ®2Θ©ΚΎ…ΪΖέΡ©”–ΘΚΧζΖέΓΔΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©ΓΔ―θΜ·―«ΧζΘ§Υυ“‘’β»ΐ÷÷œύΜΞΉιΚœ«ιΩω”–ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆΥΡ―θΜ·»ΐΧζΘ®Fe3O4Θ©Ήι≥…ΓΔΚΎ…ΪΖέΡ©A”…ΧζΖέΚΆ―θΜ·―«ΧζΘ®FeOΘ©Ήι≥…ΓΔΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…ΘΜ“ρΈΣΧζΖέΡή±Μ¥≈ΧζΈϋ“ΐΘ§ΥΡ―θΜ·»ΐΧζ“≤Ρή±Μ¥≈ΧζΈϋ“ΐΘ§Υυ“‘»γΙϊ «’βΝΫ÷÷Έο÷ Ήι≥…Θ§œ÷œσΈΣΘΚΚΎ…ΪΖέΡ©A»Ϊ≤Ω±ΜΈϋ“ΐΘΜ”…”Ύ―θΜ·―«Χζ»ή”ΎΥαΘ§ΥΡ―θΜ·»ΐΧζ≤Μ»ή”ΎΥαΘ§Υυ“‘Φ”»κΥαΘ§Ω¥ΚΎ…ΪΙΧΧε «Ζώ±Μ»ήΫβΘ§»γΙϊ≤ΩΖ÷»ήΫβΘ§ΥΒΟςΚ§”–ΥΡ―θΜ·»ΐΧζΘΜ–ΓΨξ»œΈΣΖΫΑΗΔΎ÷–ΒΟ≥ωΒΡΫα¬έ≤Μ’ΐ»ΖΥΐ»œΈΣΚ§”–ΥΡ―θΜ·»ΐΧζ ±Θ§“≤ΖΔ…ζΆ§―υΒΡœ÷œσΘΜ
ΗυΨί÷ ΝΩΒΡΦθ…ΌΝΩ «…ζ≥…«βΤχΒΡ÷ ΝΩΘ§Υυ“‘…ζ≥…«βΤχΒΡ÷ ΝΩΈΣΘΚ8g+50g-57.8g=0.2gΘ§…η“Σ…ζ≥…0.2g«βΤχΘ§–η“ΣΧζ÷ ΝΩΈΣX‘ρΘΚFe+H2SO4=FeSO4+H2Γϋ
56 2
X 0.2g
ΗυΨίΘΚ
 ΫβΒΟX=5.6gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «
ΫβΒΟX=5.6gΘ§‘ρΗΟΚΎ…ΪΖέΡ©A÷–ΧζΒΡ÷ ΝΩΖ÷ ΐ «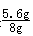 ΓΝ100%=70%Θ°
ΓΝ100%=70%Θ°Ι ¥πΑΗΈΣΘΚΘ®1Θ©ΝρΥαΆ≠»ή“ΚΓΔΙΐ¬ΥΘΜΦ”»κΉψΝΩΝρΥαΆ≠»ή“ΚΖ¥”ΠΚσΙΐ¬ΥΘ§ΜΙ”–ΚΎ…ΪΖέΡ©¥φ‘Ύ
Θ®2Θ©≤¬œκ3ΘΚΚΎ…ΪΖέΡ©A”…ΧζΖέΓΔ―θΜ·―«ΧζΚΆΥΡ―θΜ·»ΐΧζΉι≥…
| ΖΫΑΗ | Β―ι≤ΌΉς | Ω…ΡήΒΡœ÷œσ | Ϋα¬έ |
| ΔΌ | | ΚΎ…ΪΖέΡ©A»Ϊ≤Ω±ΜΈϋ“ΐ | |
| ΔΎ | | | |
| Δέ | »Γ ΝΩΚΎ…ΪΖέΡ©BΘ§Φ”»κΉψΝΩœΓ―ΈΥα Θ®Μρ’Ώ»Γ ΝΩΚΎ…ΪΖέΡ©B”Ο¥≈ΧζΈϋ“ΐΘΜΚΎ…ΪΖέΡ©≤ΩΖ÷±ΜΈϋ“ΐΘ© | ΚΎ…ΪΖέΡ©B≤ΩΖ÷»ήΫβ Θ®ΚΎ…ΪΖέΡ©B≤ΩΖ÷±ΜΈϋ“ΐΘ© | |
ΦΤΥψΘΚ70%

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ

 XΓϋ+ 2SO2Γϋ + 2H2O ‘ρ…ζ≥…ΈοXΒΡΜ·―ß ΫΈΣ ΓΘ
XΓϋ+ 2SO2Γϋ + 2H2O ‘ρ…ζ≥…ΈοXΒΡΜ·―ß ΫΈΣ ΓΘ