��Ŀ����
 ��ľ����ũ�ҷ��ϣ�������Ҫ�ɷ���̼��أ���������ء��Ȼ��صȣ���ѧ��ȤС��Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ100g��ľ�����ձ��У����ϵ���ϡ������Һ��������30gϡ����ʱ�����������ݲ�������ʱ�ձ��еIJ�����������Ϊ127.8g��̼��������ᷴӦ�Ļ�ѧ����ʽΪK2CO3+H2SO4�TK2SO4+CO2��+H2O �������ľ�ҵ������ɷֲ�����Ԫ�ء������ᷴӦ��
��ľ����ũ�ҷ��ϣ�������Ҫ�ɷ���̼��أ���������ء��Ȼ��صȣ���ѧ��ȤС��Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ100g��ľ�����ձ��У����ϵ���ϡ������Һ��������30gϡ����ʱ�����������ݲ�������ʱ�ձ��еIJ�����������Ϊ127.8g��̼��������ᷴӦ�Ļ�ѧ����ʽΪK2CO3+H2SO4�TK2SO4+CO2��+H2O �������ľ�ҵ������ɷֲ�����Ԫ�ء������ᷴӦ�������ش�
��1��������̼��CO2�������У�̼����Ԫ�ص�ԭ�Ӹ�����Ϊ
��2��̼��أ�K2CO3������Է�������Ϊ
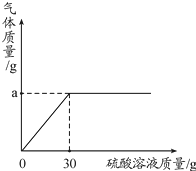
��3����ͼ��ʾ��Ӧ���̷ų��������������������Ĺ�ϵ���ߣ�����������غ㶨�����ͼ����������a����ֵ��a=
��4����ľ����Ʒ��̼��ص�������Ҫ��д��������̣���
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,��Է��������ĸ�������
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����ݻ�ѧʽ����ؼ���������������̼��̼ԭ�Ӻ���ԭ�ӵĸ����ȣ�
��2�����ݻ�ѧʽ�ļ�����Լ����̼��ص���Է���������
��3�����������غ㶨�ɿ��Լ�������������������
��4���������������������Ϸ�Ӧ�Ļ�ѧ����ʽ���Լ����̼��ص�������
��2�����ݻ�ѧʽ�ļ�����Լ����̼��ص���Է���������
��3�����������غ㶨�ɿ��Լ�������������������
��4���������������������Ϸ�Ӧ�Ļ�ѧ����ʽ���Լ����̼��ص�������
����⣺��1��������̼��CO2�������У�̼����Ԫ�ص�ԭ�Ӹ�����Ϊ1��2��
��2��̼��أ�K2CO3������Է�������Ϊ��39��2+12+16��3=138��
��3���������غ㶨�ɿ���֪������Ӧǰ����ٵ�������Ϊ���ɵĶ�����̼�����������Կ������ͼ����������a����ֵΪ��100g+30g-127.8g=2.2g��
��4����ľ����Ʒ��̼��ص�����Ϊx
K2CO3+H2SO4�TK2SO4+CO2��+H2O
138 44
x 2.2g
=
��ã�x=6.9g
�𣺲�ľ����Ʒ��̼��ص�����Ϊ6.9g��
�ʴ�Ϊ����1��1��2��
��2��138��
��3��2.2��
��4��6.9g��
��2��̼��أ�K2CO3������Է�������Ϊ��39��2+12+16��3=138��
��3���������غ㶨�ɿ���֪������Ӧǰ����ٵ�������Ϊ���ɵĶ�����̼�����������Կ������ͼ����������a����ֵΪ��100g+30g-127.8g=2.2g��
��4����ľ����Ʒ��̼��ص�����Ϊx
K2CO3+H2SO4�TK2SO4+CO2��+H2O
138 44
x 2.2g
| 138 |
| x |
| 44 |
| 2.2g |
��ã�x=6.9g
�𣺲�ľ����Ʒ��̼��ص�����Ϊ6.9g��
�ʴ�Ϊ����1��1��2��
��2��138��
��3��2.2��
��4��6.9g��
������������Ҫ����ѧ�����û�ѧ����ʽ�ͻ�ѧʽ����ؼ��㣬�������ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
�����Ŀ
ij��ѧС���ڵ����г�ʱ��������һЩ�����������Ὠ���в���ȷ���ǣ�������
| A�����������������ñ��ʣ������ü�ȩ��Һ���ݺ��ʷ��� |
| B����ë��ά�ͺϳ���ά��۲��ü��𣬽�����������ȼ�շ����� |
| C���Ͼɵ�����ⶪ���Ի�������Ⱦ�����齫����ղ����д��� |
| D���߲˱������������ʣ������װʱ����һС��������������������ˮ�����ܷ� |
������̼����������Ȼ�������������ȱ�ٵ��������壬���ǵ���ͬ���ǣ�������
| A��������� |
| B�����ǹ�����õ�ԭ�� |
| C�����ܹ����� |
| D������ |