��Ŀ����
��2011?������ģ�⣩2011������3?15�����ع⣺�ӱ�ʡ������ʯ��ׯ�ȵ���ijЩ��ҵΥ�����������涨���û��յ�������ֽ�����������о��ռ��ӫ�������������������ʲ������ͽ�ֽ��������ѧ�ռ�֪ʶ�ش��������⣺
��1�����ռ���������Ƶ��׳ƣ���ͨ���ֱ���Ϊ
��2���ɷ��й���˵�����վ���ָ������
��ֽƬ�ϡ����ñ������ס��С�ձ��С�����ƽ���̡�����ƽ����
��3���������ƹ����к�ǿ����ˮ�ԣ�����ʯ�Ұ�������Ϻ��Ϊ��ʯ�ң��dz��õ�����������������ѧ����֪ʶ��д����ʯ����ˮ��Ӧ�Ļ�ѧ����ʽ��
��CO2 ��CO ��H2 ��O2 ��SO2
��4����ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ƹ���ijɷ֣��������й�ʵ�飮����������һ���������̽�����
[�Թ������]
�����ȫ����NaOH��
�����ȫ����Na2CO3��
�������NaOH��Na2CO3����
[ʵ�����]
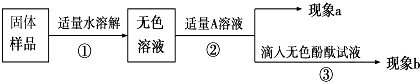
[������]
��������aΪ�����ݲ�����������A��Һ��
����A��Ca��OH��2��Һ������a�а�ɫ����������bΪ��ɫ��̪��Һ���ɫ�����ɫ����Ϊ
����A��CaCl2��Һ����ʵ������aΪ
[��˼]���õ��������Ʊ��ʵ�ԭ���ǣ��û�ѧ����ʽ��ʾ��
��1�����ռ���������Ƶ��׳ƣ���ͨ���ֱ���Ϊ
��������
��������
����2���ɷ��й���˵�����վ���ָ������
��ʴ
��ʴ
��ǿ����ѧʵ������Ҫ��ȡһ���������������ƣ�Ӧ�����ڢ�
�ڢ�
����ֽƬ�ϡ����ñ������ס��С�ձ��С�����ƽ���̡�����ƽ����
��3���������ƹ����к�ǿ����ˮ�ԣ�����ʯ�Ұ�������Ϻ��Ϊ��ʯ�ң��dz��õ�����������������ѧ����֪ʶ��д����ʯ����ˮ��Ӧ�Ļ�ѧ����ʽ��
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
���������岻���ü�ʯ�Ҹ�������٢�
�٢�
�� ��CO2 ��CO ��H2 ��O2 ��SO2
��4����ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ƹ���ijɷ֣��������й�ʵ�飮����������һ���������̽�����
[�Թ������]
�����ȫ����NaOH��
�����ȫ����Na2CO3��
�������NaOH��Na2CO3����
[ʵ�����]

[������]
��������aΪ�����ݲ�����������A��Һ��
ϡ����
ϡ����
��˵�����������Ѿ����ʣ������ݲ����ķ�Ӧ�Ļ�ѧ����ʽ��Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
������A��Ca��OH��2��Һ������a�а�ɫ����������bΪ��ɫ��̪��Һ���ɫ�����ɫ����Ϊ
CaCO3
CaCO3
���ѧʽ������ʵ������
����
����ܡ����ܡ���˵����Ʒ����NaOH������A��CaCl2��Һ����ʵ������aΪ
�а�ɫ��������
�а�ɫ��������
����������bΪ��ɫ��̪��Һ����ɫ
��ɫ��̪��Һ����ɫ
�������������[��˼]���õ��������Ʊ��ʵ�ԭ���ǣ��û�ѧ����ʽ��ʾ��
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
����������1�������������Ƶ��׳ƽ��н��
��2�������������ƾ���ǿ�ҵĸ�ʴ�Խ��н��
��3��������ʯ����ˮ��Ӧ���������Լ���ʯ�����ڼ��Ը�������н��
��4���������������������̼������Ӧ����ˮ��̼���ƣ������������տ����е�ˮ�ּ�������̼�����ʣ����Ծݴ˽����⣮
���������Ʊ��ʺ�IJ���Ϊ̼���ƣ���̼������ҺҲ�ʼ��ԣ�����ʹ��̪��죬���Բ����÷�̪��Һ����Na0H�Ƿ���ʣ����Ծݴ˽����⣮
�۸����������Ƶ����ʽ��з�������������¶���ڿ��������������̼��Ӧ����̼���ƶ����ʣ���Ӧ�ij̶Ȳ�ͬ������ʵij̶Ȳ�ͬ������̼����ʹ�õ��Ǽ��ữ���ķ�������뺬�п����Ը����ӡ������ӵķ�����Ҫ֤�������������ƣ�����Ҫ��ȥ̼�����ٽ��м��飬�Ҽ�����Լ���̼���Ʒ�Ӧ���������Լ��Ե����ʣ�
[��˼]�����������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ���н��
��2�������������ƾ���ǿ�ҵĸ�ʴ�Խ��н��
��3��������ʯ����ˮ��Ӧ���������Լ���ʯ�����ڼ��Ը�������н��
��4���������������������̼������Ӧ����ˮ��̼���ƣ������������տ����е�ˮ�ּ�������̼�����ʣ����Ծݴ˽����⣮
���������Ʊ��ʺ�IJ���Ϊ̼���ƣ���̼������ҺҲ�ʼ��ԣ�����ʹ��̪��죬���Բ����÷�̪��Һ����Na0H�Ƿ���ʣ����Ծݴ˽����⣮
�۸����������Ƶ����ʽ��з�������������¶���ڿ��������������̼��Ӧ����̼���ƶ����ʣ���Ӧ�ij̶Ȳ�ͬ������ʵij̶Ȳ�ͬ������̼����ʹ�õ��Ǽ��ữ���ķ�������뺬�п����Ը����ӡ������ӵķ�����Ҫ֤�������������ƣ�����Ҫ��ȥ̼�����ٽ��м��飬�Ҽ�����Լ���̼���Ʒ�Ӧ���������Լ��Ե����ʣ�
[��˼]�����������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ���н��
����⣺��1���������Ƶ��׳ƣ��ռ�������ƣ�
��2���������ƾ���ǿ�ҵĸ�ʴ�ԣ���ѧʵ������Ҫ��ȡһ���������������ƣ�Ӧ�����ñ������ס��С�ձ��к���ƽ���̣�
��3����ʯ����ˮ��Ӧ�������ƣ���Ӧ�Ļ�ѧ����ʽ�ǣ�CaO+H2O�TCa��OH��2����ʯ�����ڼ��Ը���������ܸ����������壬�ʲ��ܸ��������̼�Ͷ�������
��4�����������Ʊ������ɵ���̼���ƣ��������A��������ݣ��������������ᣬ������ϡ���ᣬ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��������������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʰ�ɫ������̼��ƣ��仯ѧʽΪ��CaCO3�����ɵ�����������ʹ��̪��Һ��죬�ʲ���ȷ��ԭ�������Ƿ����������ƣ�
����ȫ����̼���ƣ��������Ȼ�������̼���Ʒ�Ӧ����̼��ư�ɫ�������Ȼ��ƣ����ɵ��Ȼ��Ƶ�ˮ��Һ�����ԣ�����ʹ��̪��Һ��죬��������aΪ������ɫ����������bΪ��̪����ɫ��
[��˼]�������������տ����е�ˮ�����Ϳ����еĶ�����̼����̼���ƺ�ˮ����ˣ�������Ӵ�������������Һ����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
�ʴ�Ϊ��
��1���������ƣ�
��2����ʴ���ڢۣ�
��3��CaO+H2O�TCa��OH��2���٢ݣ�
��4����ϡ��� Na2CO3+2HCl�T2NaCl+H2O+CO2����
��CaCO3�����ܣ�
���а�ɫ�������ɣ���ɫ��̪��Һ����ɫ��
[��˼]��2NaOH+CO2�TNa2CO3+H2O��
��2���������ƾ���ǿ�ҵĸ�ʴ�ԣ���ѧʵ������Ҫ��ȡһ���������������ƣ�Ӧ�����ñ������ס��С�ձ��к���ƽ���̣�
��3����ʯ����ˮ��Ӧ�������ƣ���Ӧ�Ļ�ѧ����ʽ�ǣ�CaO+H2O�TCa��OH��2����ʯ�����ڼ��Ը���������ܸ����������壬�ʲ��ܸ��������̼�Ͷ�������
��4�����������Ʊ������ɵ���̼���ƣ��������A��������ݣ��������������ᣬ������ϡ���ᣬ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��������������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʰ�ɫ������̼��ƣ��仯ѧʽΪ��CaCO3�����ɵ�����������ʹ��̪��Һ��죬�ʲ���ȷ��ԭ�������Ƿ����������ƣ�
����ȫ����̼���ƣ��������Ȼ�������̼���Ʒ�Ӧ����̼��ư�ɫ�������Ȼ��ƣ����ɵ��Ȼ��Ƶ�ˮ��Һ�����ԣ�����ʹ��̪��Һ��죬��������aΪ������ɫ����������bΪ��̪����ɫ��
[��˼]�������������տ����е�ˮ�����Ϳ����еĶ�����̼����̼���ƺ�ˮ����ˣ�������Ӵ�������������Һ����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
�ʴ�Ϊ��
��1���������ƣ�
��2����ʴ���ڢۣ�
��3��CaO+H2O�TCa��OH��2���٢ݣ�
��4����ϡ��� Na2CO3+2HCl�T2NaCl+H2O+CO2����
��CaCO3�����ܣ�
���а�ɫ�������ɣ���ɫ��̪��Һ����ɫ��
[��˼]��2NaOH+CO2�TNa2CO3+H2O��
������������Ҫ������ѧ�����û�ѧ֪ʶ�����������о�����������������������漰��֪ʶ��ȽϷ�ɢ���ڿα���Ҳ�ȽϷ�ɢ������ƽʱѧϰҪ��������������

��ϰ��ϵ�д�
�����Ŀ
 ��2011?������ģ�⣩��ͼ��ʾ��һ�������·�����ij��ѧ��Ӧ��������˵����ȷ���ǣ�������
��2011?������ģ�⣩��ͼ��ʾ��һ�������·�����ij��ѧ��Ӧ��������˵����ȷ���ǣ�������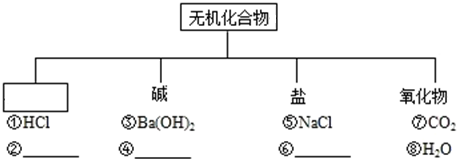
 ��2011?������ģ�⣩��ͼΪA��B��C����Ԫ�ص����ӽṹʾ��ͼ���ش��������⣺
��2011?������ģ�⣩��ͼΪA��B��C����Ԫ�ص����ӽṹʾ��ͼ���ش��������⣺