题目内容
下列是酸、碱、盐的一些用途,写出相关的化学方程式:
(1)生石灰做干燥剂: ,该干燥剂能否干燥CO2 .
(2)用含氢氧化铝的胃舒平治疗胃酸过多的患者 .还可以用 (填一种物质化学式)治疗胃酸过多.
(3)某些工厂用稀硫酸清洗铁制品表面的铁锈(主要成分氧化铁): ;溶液由无色变为 色.
(4)用烧碱溶液吸收工业废气中的SO2,以减少污染 .该反应 (是不是)复分解反应.
(5)将少量硫酸铵晶体与熟石灰混合研磨: ;研磨用的器皿是: .
(1)生石灰做干燥剂:
(2)用含氢氧化铝的胃舒平治疗胃酸过多的患者
(3)某些工厂用稀硫酸清洗铁制品表面的铁锈(主要成分氧化铁):
(4)用烧碱溶液吸收工业废气中的SO2,以减少污染
(5)将少量硫酸铵晶体与熟石灰混合研磨:
考点:书写化学方程式、文字表达式、电离方程式,反应类型的判定
专题:化学用语和质量守恒定律
分析:(1)生石灰是氧化钙的俗称,能和水反应生成氢氧化钙,氢氧化钙能够二氧化碳反应生成碳酸钙和水;
(2)氢氧化铝和稀盐酸反应生成氯化铝和水,碳酸氢钠能和稀盐酸反应生成氯化钠、水和二氧化碳;
(3)氧化铁和稀硫酸反应生成硫酸铁和水;
(4)氢氧化钠和二氧化硫反应生成亚硫酸钠和水;
(5)硫酸铵和氢氧化钙反应生成硫酸钙、水和氨气.
(2)氢氧化铝和稀盐酸反应生成氯化铝和水,碳酸氢钠能和稀盐酸反应生成氯化钠、水和二氧化碳;
(3)氧化铁和稀硫酸反应生成硫酸铁和水;
(4)氢氧化钠和二氧化硫反应生成亚硫酸钠和水;
(5)硫酸铵和氢氧化钙反应生成硫酸钙、水和氨气.
解答:解:(1)氧化钙和水反应的化学方程式为:CaO+H2O═Ca(OH)2,氧化钙不能干燥二氧化碳,因为氢氧化钙和二氧化碳能够反应.
故填:CaO+H2O═Ca(OH)2;不能,因为 Ca(OH)2+CO2═CaCO3↓+H2O.
(2)氢氧化铝和稀盐酸反应的化学方程式为:Al(OH)3+3HCl═AlCl3+3H2O,还可以用NaHCO3治疗胃酸过多.
故填:Al(OH)3+3HCl═AlCl3+3H2O;NaHCO3.
(3)氧化铁和稀盐酸飞一定化学方程式为:Fe2O3+3H2SO4═Fe2(SO4)3+3H2O,因为硫酸铁溶液是黄色的,所以溶液由无色变为黄色.
故填:Fe2O3+3H2SO4═Fe2(SO4)3+3H2O;黄.
(4)氢氧化钠和二氧化硫反应的化学方程式为:2NaOH+SO2═Na2SO3+H2O,因为不是两种化合物简单地交换成分,所以该反应不属于复分解反应.
故填:2NaOH+SO2═Na2SO3+H2O;不是.
(5)硫酸铵和氢氧化钙反应的化学方程式为:(NH4)2SO4+Ca(OH)2═CaSO4+2H2O+2NH3↑,研磨用的器皿是研钵.
故填:(NH4)2SO4+Ca(OH)2═CaSO4+2H2O+2NH3↑;研钵.
故填:CaO+H2O═Ca(OH)2;不能,因为 Ca(OH)2+CO2═CaCO3↓+H2O.
(2)氢氧化铝和稀盐酸反应的化学方程式为:Al(OH)3+3HCl═AlCl3+3H2O,还可以用NaHCO3治疗胃酸过多.
故填:Al(OH)3+3HCl═AlCl3+3H2O;NaHCO3.
(3)氧化铁和稀盐酸飞一定化学方程式为:Fe2O3+3H2SO4═Fe2(SO4)3+3H2O,因为硫酸铁溶液是黄色的,所以溶液由无色变为黄色.
故填:Fe2O3+3H2SO4═Fe2(SO4)3+3H2O;黄.
(4)氢氧化钠和二氧化硫反应的化学方程式为:2NaOH+SO2═Na2SO3+H2O,因为不是两种化合物简单地交换成分,所以该反应不属于复分解反应.
故填:2NaOH+SO2═Na2SO3+H2O;不是.
(5)硫酸铵和氢氧化钙反应的化学方程式为:(NH4)2SO4+Ca(OH)2═CaSO4+2H2O+2NH3↑,研磨用的器皿是研钵.
故填:(NH4)2SO4+Ca(OH)2═CaSO4+2H2O+2NH3↑;研钵.
点评:书写化学方程式要注意四步:一是反应物和生成物的化学式要正确;二是要遵循质量守恒定律,即配平;三是要有必要的条件;四是看是否需要“↑”或“↓”.

练习册系列答案
 金牌教辅培优优选卷期末冲刺100分系列答案
金牌教辅培优优选卷期末冲刺100分系列答案
相关题目
除去粗盐中的难溶性杂质,可通过以下的实验步骤来完成.下列操作中正确的是( )
A、 溶解 |
B、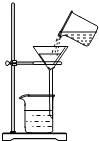 过滤 |
C、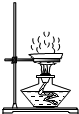 蒸发 |
D、 称量精盐 |