��Ŀ����
��4 �֣���ֽ���ҹ��Ŵ��Ĵ���֮һ������Ч���ƶ������������ķ�չ��
�Ż���ũ����Ľո���Ϊ��ֽԭ�ϣ����Լ��ٷ��սոѴ������̳���Ⱦ���ոѵ���Ҫ �ɷ�֮һ����ά��[��ѧʽ (C6H10O5)n]������������������Ԫ����______�� ��ά������______��ѡ��л����������������֮һ����
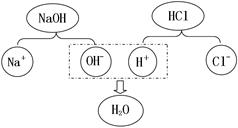
����ֽ�����л���������� NaOH �ķ�ˮ��Сӱͬѧ���� pH ��ֽ���Բⶨ��ˮ���ȣ��ⶨ�ľ��巽���� ��
��Ϊ�����÷�ˮ�Ի�������Ⱦ�����ϡ���ᡢ����ͭ��̼�������ҩƷ��ѡ��һ��ҩƷ������ȥ��ˮ�е�NaOH������д���ó��ӷ�Ӧ�Ļ�ѧ����ʽ�� ��
�� ��Ԫ�� �л��� �� 2NaOH + H2SO4 = Na2SO4 + 2H2O
�� ��pH��ֽ���ڽྻ����İ״�Ƭ�������ϣ��ýྻ����IJ�����պȡ����Һ��
����pH��ֽ�ϣ�Ѹ�������ɫ�����ж��ա�
���������������1������ά���У���Ԫ�ص����ԭ��������࣬�������������ά�����к���̼Ԫ�����л��
��2���ⶨ��ҺPH�ķ������ò�����պȡ��������Һ����PH��ֽ�ϣ��ٽ���ʾ����ɫ�����ɫ�����ж��գ�
��3��ϡ���ᡢ����ͭ��̼�������ҩƷ��ֻ��ϡ���������������Ʒ����кͷ�Ӧ��2NaOH + H2SO4 = Na2SO4 + 2H2O
���㣺�й���Է��������ļ��㡢PH�IJⶨ���кͷ�Ӧ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�Ϊ���ѧѧ����ȡ�õijɾ��Լ������������Ĺ��ף����Ϲ���2011�궨Ϊ�����ʻ�ѧ�ꡱ����ش��������⣺
��1�����ϵķ�չ�ƶ����Ľ��������в������ںϳɲ��ϵ��� ������ţ�
| A������ϩ���� | B������� | C���� | D����ë |
��3�������ڻ�ѧ���������� �ʣ�������Ҫ�����Ǵٽ�ֲ��������ʹֲ��ҶɫŨ�̡�