��Ŀ����
����Ŀ���ҹ����ڡ���ȼ�������ɼ�����ȡ���ش�ͻ�ƣ��ӡ���ȼ�����пɻ�ü��顣
(1)�����ڿ�����ȼ�յ�������_______________����ѧ����ʽ��_____��
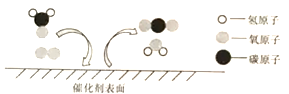
(2)���۽�һ����ʶ��ѧ��Ӧ����ͼ��һ���������������ַ�Ӧ����ʾ��ͼ��

�ٸû�ѧ��Ӧ���������仯��������______(����ĸ���)��
A��̼ԭ�ӡ�����B����ԭ�ӡ�����C����ԭ��
D��������ӡ���E��������
�ڸ���ʾ��ͼ���ֳ���Ӧǰ�����������������Ͽ�����____(����ĸ���)��
A��16 g�����64 g����
B��8 g�����32 g����
C��20 g�����64 g����
D��16 g�����96 g����
���𰸡� ������ɫ���棬�ų��������� CH4��2O2![]() CO2��2H2O ABC D
CO2��2H2O ABC D
�����������⿼���˼����ȼ�գ�ȼ�չ��������ı仯�������ȡ�
��1�������ڿ�����ȼ�ղ����˵���ɫ�Ļ��棬�ų��������ȣ������ڿ�����ȼ�������˶�����̼��ˮ����ѧ����ʽ�ǣ�CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��2������һ���������������ַ�Ӧ����ʾ��ͼ�����ı仯��֪���û�ѧ��Ӧ�в������仯��������̼ԭ�ӡ���ԭ�ӡ���ԭ������ѡABC��
������ʾ��ͼ��֪��ͼ�м������������Ӹ�����Ϊ1��3�����������ǣ�16����32��3��=16��96����Ӧǰ�����������������Ͽ�����16 g�����96 g��������ѡD��

����Ŀ��ijС���ʯ��ʯ�������գ��������պ����ɷֽ���̽����
��1��д���˷�Ӧ�Ļ�ѧ����ʽ_______________________�� ��2�����ݻ�ѧ����ʽ���в��롣���������ɷ֣� I. ֻ�������ƣ�II. ֻ��̼��ƣ�III. _____________ ��3�����ұ���λͬѧ�ֱ���������ַ���̽�����պ����ɷ֡� ������ʾ����CaO+2HCl��CaCl2+H2O�� ��Ca(OH)2+2HCl��CaCl2+2H2O ���Ȼ�����Һ�����ԡ� | |||||
������� | ���������� | ʵ����� | ���� | ||
����һ |
| ���������� һ������_______ | �û�ѧ����ʽ�����ж�����_______�� | ||
������ |
| ��ͬѧ�ó� ����II���� | ��ͬѧ��Ʒ����Ƿ������������______�� | ||
������ |
| ����III���� | |||