��Ŀ����
ij����X��H2��CO�е�һ�ֻ�������ɣ�ij��ѧ��ȤС��Ը�����X����ɽ���̽����
��������⡿������X��ʲô������ɣ�
��1����������衿�����ֻ��������
�����ֻ��һ����̼��
����� ��
���ṩ��Ϣ����H2��CO�е�һ�ֻ�������ɵ��������������а�����ȼ�ա�
 |
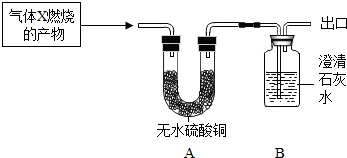
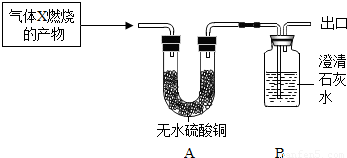
�����ʵ�顿��������X��������ȼ�յIJ�������ͨ��װ��A��B������װ��A��B�з����ı仯������Ʋ���������ɡ�
��2������������ۡ�
�����۲쵽A�а�ɫ����������B����![]() �仯������ ������A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
�仯������ ������A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
�����۲쵽A���ޱ仯��B�г��ֻ�������������X��________��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________���ӻ����Ƕȿ�����Ϊ��ͼʵ����Ľ��Ĵ�ʩ��
_____________________________________________��
��3������˼��
��������ij��������ȫȼ�գ������������ͨ������ʵ��װ��A��װ��B������װ��A�а�ɫ����������B�г��ֻ���������ô���������� ����дһ�����ʵĻ�ѧʽ�����ƣ���
��������������X��ͨ�����ȵ�����ͭ����ͨ��װ��A��װ��B��Ҳ��̽��������X����ɣ�
��д��һ����̼������ͭ��Ӧ�Ļ�ѧ����ʽ_________________________________��
��1��������룺������һ����̼�Ļ������
��2����������ۣ��� �� CuSO4 +5H2O CuSO4·5H2O
�� CO�� CO2 + Ca��OH��2 CaCO3��+ H2O ����β����ȼ������ռ���
��3����˼���ټ��飨CH4������Ȳ���ƾ��� �� CO+CuO Cu+CO2

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
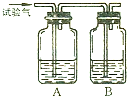 ��2006?ƽ������ģ��ij����X�������������������H2��CO��CH4�е�һ�ֻ�����ɣ���Xȼ�գ���ȼ�պ����ɵ���������ͨ������ͼ��ʾ��A��B��������ƿ���Իش�
��2006?ƽ������ģ��ij����X�������������������H2��CO��CH4�е�һ�ֻ�����ɣ���Xȼ�գ���ȼ�պ����ɵ���������ͨ������ͼ��ʾ��A��B��������ƿ���Իش�