��Ŀ����
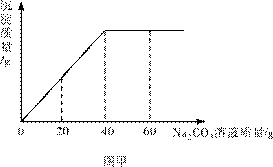
ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ�![]() ��������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ����ʾ����֪��BaCl2��Na2CO3��BaCO3����2NaCl ����㣺
��������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ����ʾ����֪��BaCl2��Na2CO3��BaCO3����2NaCl ����㣺
��1������26.5%��Na2CO3��Һ80g����ҪNa2CO3���� g��
��2��BaCl2��Һ���������������Ƕ��٣���д��������̣����������0.1%��
(1) 21.2g ��2�֣�
�⣺(2)��ͼ���л�֪����BaCl2��ȫת��Ϊ����ʱ����Na2CO3��Һ������Ϊ40g��
��BaCl2������Ϊ![]() x���� --------------------------------------��1�֣�
x���� --------------------------------------��1�֣�
BaCl2��Na2CO3��BaCO3����2NaCl
208����106������������������������
x������40g��26.5%
![]() ��
��
��ã�x��20.8g��
��BaCl2��Һ������������������20.8g/150g��x 100%=13.9%����
---------��1�֣�
�𣺸�BaCl2��Һ��������������Ϊ13.9%��
�����������������Ҳ���֣�

��ϰ��ϵ�д�
�����Ŀ
 ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ����֪��BaCl2+Na2CO3=BaCO3��+2NaCl
ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ����֪��BaCl2+Na2CO3=BaCO3��+2NaCl  ��2012?������ģ�⣩ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ����ʾ������㣺
��2012?������ģ�⣩ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ����ʾ������㣺 ��2012?�߰���һģ��ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��100g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ����֪��BaCl2+Na2CO3=BaCO3��+2NaCl
��2012?�߰���һģ��ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��100g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ����֪��BaCl2+Na2CO3=BaCO3��+2NaCl  ��2013?�㶫ģ�⣩ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ��
��2013?�㶫ģ�⣩ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ��ʾ��