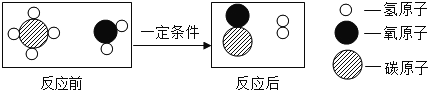
��Ŀ����
����Ŀ����������Դ�����ǵ�����ᱸ�ܹ�ע���ȵ㡣
��1��Ŀǰ�����Ի�ʯȼ��Ϊ��Ҫ��Դ,�����Ļ�ʯȼ�ϰ���ú��______����Ȼ����
��2�������ᳫ����̼����������Ҫ��Ϊ�˼��ٺ�̼��Դ���ģ��Ӷ�����̼��������ŷš��������Ҫע�ؿ�����ʹ������Դ���д�����������Դ��_____��дһ�ּ��ɣ���
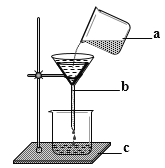
��3����ʯȼ��ȼ�ղ�������CO2��ȥ���ҹ���Χ�ڳ���������������PM2.5�����������������Ԫ����֮һ��PM 2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ�ƿ���ο����������Ϊ�ܲ���PM2.5����________ ��
A�������ض�ʱ��ˮ���� B������ȼ�ű���
C��У�������ѻ��������͵ط��� DΪ������У��ᳫ���������ʹ��˽�ҳ�
��4��������CO2���������ǵ�������ЧӦ������Ҫԭ����Ȼ��������CO2����Ҫ;����____________________��
��5������1 g CH4��ȫȼ�ղ���CO2������m = ___________ g�������ȷ��0.01 g����
��6�����±����ݷ�������ú��ȣ�����Ȼ����ȼ�ϵ��ŵ���_____________��дһ�㼴�ɣ�
1g������ȫȼ�ղ���CO2������ | 1g������ȫȼ�շų������� | |
CH4 | m | 56 kJ |
C | 3.67 g | 32 kJ |
���𰸡�ʯ�� ���� �����ܡ�̫���ܡ������ܡ���ϫ�ܵȺ������ɣ� B C D ֲ��Ĺ������ 2.75 ��ͬ������CH4��ȫȼ�ղ�����CO2��ú���٣�����ͬ������CH4��ȫȼ�ճ���������ú�Ķࣩ
��������
��1������ʯȼ�ϣ�ú��ʯ�͡���Ȼ����
��2���д�����������Դ�У����ܡ����ܡ�̫���ܡ������ܡ���ϫ�ܵ�
��3��A �������ض�ʱ��ˮ�������Լ�С����PM2.5
B ������ȼ�ű��ڲ�������������������PM2.5Ũ�ȣ�
C ��У�������ѻ��������͵ط��գ�������������������PM2.5Ũ�ȣ�
D Ϊ������У��ᳫ���������ʹ��˽�ҳ�������������β���ŷţ�����PM2.5Ũ�ȣ�
��4��ֲ��Ĺ�����ÿ������ն�����̼����������
��5���⣺�����������̼������Ϊx��
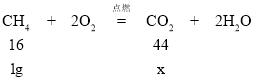
![]() =
=![]() ��֮�ã�x=2.75g
��֮�ã�x=2.75g
��6����ͬ������CH4��ȫȼ�ղ�����CO2��ú���٣���ͬ������CH4��ȫȼ�ճ���������ú�Ķ�

����Ŀ����100gBaCl2��Һ�еμ�Na2SO4��Һ����ȫ��Ӧ����Ӧ���������ɳ�����������μ�Na2SO4��Һ��������ϵ�����ʾ������Ӧ�Ļ�ѧ����ʽ��BaCl2+Na2SO4=BaSO4��+2NaCl��������㣺
�μ�Na2SO4��Һ������/g | 10 | 20 | 30 | 40 |
���ɳ���������/g | 2.33 | 4.66 | 6.99 | 6.99 |
��1��Na2SO4����Է�������Ϊ______________��
��2��ǡ����ȫ��Ӧʱ����BaSO4������Ϊ ______g
��3��BaCl2��Һ�����ʵ���������___________��д��������̣�