��Ŀ����
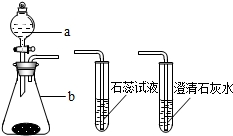
 14����1����Ũ������Լ�ƿʱ�������Լ�ƿ�ڷ�����������
14����1����Ũ������Լ�ƿʱ�������Լ�ƿ�ڷ��������������������İ���
����һ��������Ũ����ʢ���ձ��У����ڷ�����ʵ���Ұ�ȫ֮����Ũ����������ͷ���������ϵ�����ߣ���ͼ����ȷ������
����д���ס����ҡ��������ŷ������������ӣ�Ũ��������������仯����Ҫԭ���ǣ�Ũ������лӷ���
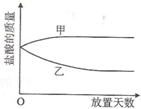
����2������10%������IJ����У���ϡ�� �ڼ��� ��װƿ������ǩ ����ȡŨ�����ˮ��
����ȷ��˳��Ϊ
�ڢܢ٢�
������ţ�����3����Ũ����ϡ�ͺ������ȥ������Ʒ��������⣨��Ҫ�ɷ�ΪFe2O3������Ӧ�Ļ�ѧ����ʽ��
Fe2O3+6HCl=FeCl3+3H2O
����������1������Ũ����Ļӷ��Ժ����������������
��2������������Һ����������������
��3���������ܺͽ��������ﷴӦ���
��2������������Һ����������������
��3���������ܺͽ��������ﷴӦ���
����⣺��1��Ũ������лӷ��ԣ��ڿ����л���������İ������ӷ����Ȼ������壬����Һ��������������С���ʣ�1���𰸣����������İ��� �� Ũ������лӷ���
��2��������Һ�IJ���������㡢��ȡ���ܽ�ϡ�͡�װƿ���ʴ𰸣��ڢܢ٢�
��3����������ϡ���ᷴӦ�����Ȼ�����ˮ���ʴ𰸣�Fe2O3+6HCl=FeCl3+3H2O
��2��������Һ�IJ���������㡢��ȡ���ܽ�ϡ�͡�װƿ���ʴ𰸣��ڢܢ٢�
��3����������ϡ���ᷴӦ�����Ȼ�����ˮ���ʴ𰸣�Fe2O3+6HCl=FeCl3+3H2O
�����������������������ʵ�����Ļ�ѧ���ʽ�����ȫ��Ŀ��飬����֪ʶ��ע��֪ʶ�����ú�����ʵ�ʵ���ϵ��

��ϰ��ϵ�д�
�����Ŀ