��Ŀ����
���������ڳ������������̼��Ӧ����̼���ƺ�������������ߺ�DZˮͧ�п��ù�������Na2O2��Ϊ��������ij��ȤС���ͬѧ�Դ˺ܸ���Ȥ���������ͼ��ʾ��ʵ�顣
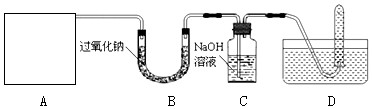
��1��A�Ƕ�����̼����ķ���װ�ã�װ���װ��ʱ��Ӧѡ�õ��������ô����ܵ�˫����Ƥ���⣬����Ҫ�������У������ƣ�_______��_______��
��2����D��ʾ�ķ����ռ���������������___________________������Dװ���Թ����ռ����������Ƿ������ķ������������ǣ���D�����弯�����Թ��Ƴ�ˮ�棬Ȼ��______________��
��3��C���������Ƶ�����������û�в��뷴Ӧ��CO2�����û���������װ�ã����ܵ��µĺ����_________________________��
��4����д�����������ڳ������������̼��Ӧ�Ļ�ѧ����ʽ_________________����˵˵�������DZˮͧ�ù���������Ϊ�������������ŵ���__________________��
��2����D��ʾ�ķ����ռ���������������___________________������Dװ���Թ����ռ����������Ƿ������ķ������������ǣ���D�����弯�����Թ��Ƴ�ˮ�棬Ȼ��______________��
��3��C���������Ƶ�����������û�в��뷴Ӧ��CO2�����û���������װ�ã����ܵ��µĺ����_________________________��
��4����д�����������ڳ������������̼��Ӧ�Ļ�ѧ����ʽ_________________����˵˵�������DZˮͧ�ù���������Ϊ�������������ŵ���__________________��
��1����ƿ������©��
��2��������������ˮ���ô����ǵ�ľ�������Թ��У�ľ����ȼ��������������
��3��Ӱ�������ļ���
��4��2Na2O2+2CO2��2Na2CO3+O2�����պ��������Ķ�����̼��ͬʱ��������
��2��������������ˮ���ô����ǵ�ľ�������Թ��У�ľ����ȼ��������������
��3��Ӱ�������ļ���
��4��2Na2O2+2CO2��2Na2CO3+O2�����պ��������Ķ�����̼��ͬʱ��������

��ϰ��ϵ�д�
�����Ŀ