��Ŀ����
��ͭ��һ����Ҫ�Ľ������ϣ��ҹ�Լ���ϱ����Ϳ�ʼұ����ͭ����ͭ��ͭ��п�ĺϽ������������������������������ճ���Ʒ��ȷ��ȡ20g��ͭм��Ʒ����ʢ��200gϡ������ձ��У�ǡ����ȫ��Ӧ������ձ������ʵ���������ʱ��Ĺ�ϵ��ͼ��ʾ����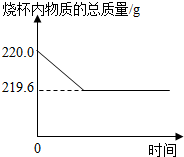 ���ܽ���Բ��ƣ���
���ܽ���Բ��ƣ���
�Լ��㣺
��1������������������
��2���û�ͭм��Ʒ��ͭ������������
��3������ϡ������������������Ƕ��٣���д��������̣�
 ���ܽ���Բ��ƣ���
���ܽ���Բ��ƣ����Լ��㣺
��1������������������
0.4
0.4
g����2���û�ͭм��Ʒ��ͭ������������
��3������ϡ������������������Ƕ��٣���д��������̣�
��������1�����������غ㶨�ɿ��������ձ������ʵ�����֮���������������������
��2�������������������÷���ʽ���������Ӧ��п���������Ӷ����������ͭм��Ʒ��ͭ������������
��3����������������Ҳ�������Ӧ��ϡ����������������������ϡ�������������������
��2�������������������÷���ʽ���������Ӧ��п���������Ӷ����������ͭм��Ʒ��ͭ������������
��3����������������Ҳ�������Ӧ��ϡ����������������������ϡ�������������������
����⣺��1����ͭ�е�ͭ�������ᷴӦ������������п����ϡ���ᷴӦ�������������������غ㶨�ɿ��������ձ������ʵ�����֮�������������������������ͼ�����ݿ�֪������Ϊ220g-219.6g=0.4g��
��2���跴Ӧ��п��������x�������������y
Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.4g
=
=
x=13g y=19.6g
�ʸû�ͭм��Ʒ��ͭ����������Ϊ
��100%=35%
��3��������ϡ�������������������
��100%=9.8%
�ʴ�Ϊ����1��0.4����2����2���û�ͭм��Ʒ��ͭ����������Ϊ35%����3������ϡ�������������������9.8%��
��2���跴Ӧ��п��������x�������������y
Zn+H2SO4=ZnSO4+H2��
65 98 2
x y 0.4g
| 65 |
| x |
| 98 |
| y |
| 2 |
| 0.4g |
x=13g y=19.6g
�ʸû�ͭм��Ʒ��ͭ����������Ϊ
| 20g-13g |
| 20g |
��3��������ϡ�������������������
| 19.6g |
| 200g |
�ʴ�Ϊ����1��0.4����2����2���û�ͭм��Ʒ��ͭ����������Ϊ35%����3������ϡ�������������������9.8%��
�����������ѶȲ��Ǻܴ����������غ㶨���ɷ�Ӧǰ���ձ������ʵ��������IJ��������������������ǽ�����ͻ�ƿڣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ