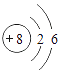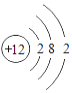��Ŀ����
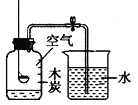
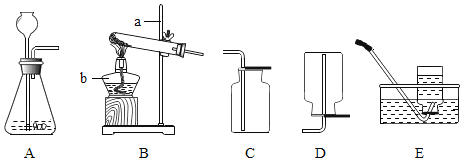
����Ŀ��������ҵ�Ļ�����������ʾ��ͼ��ͼ���ش���������
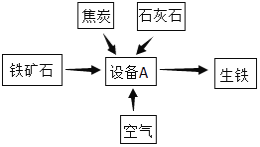
��1���豸A��������________��
��2���Գ�����Ϊԭ���ڸ�������һ����̼��Ӧ��ȡ�����Ļ�ѧ����ʽΪ________��
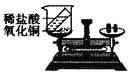
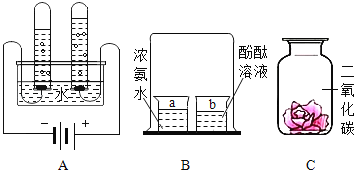
��3��ʳƷ��װ���г���װ�����ۺ���ʯ�ҵ�С����С��ͬѧ�����л�ѧ��������ijС����ʢ�ŵ������ۻ�����ʯ�ң�ȡ��Ʒ�������м�⣬������ȷ����________��
A����ϡ���ᣬ�����ݲ���Ϊ���ۣ������ݲ���Ϊ��ʯ��
B����ˮ�У�û�仯Ϊ���ۣ����ȡ�Һ������Ϊ��ʯ��
C��������ͭ��Һ�У��к�ɫ���ʲ���Ϊ���ۣ�����ɫ��������Ϊ��ʯ��
��4��ұ��1680t������3%����������Ҫ��Fe3O492.8%�Ĵ������������________t��
���𰸡���¯ 3CO+Fe2O3![]() 2Fe+3CO2 ABC 2425
2Fe+3CO2 ABC 2425
��������
��1���������豸��Ҫ�ǣ���¯��
��2�����������Ҫ�ɷ�ΪFe2O3����CO�������������������̼����Ӧ�Ļ�ѧ����ʽΪ��3CO+Fe2O3 ![]() 2Fe+3CO2��
2Fe+3CO2��
��3��A ������ϡ���ᷴӦ�����Ȼ������������������ݲ�������ʯ����ϡ���ᷴӦ�����Ȼ��ƺ�ˮ�������ݲ�������A��ȷ��
B ������ˮ����Ӧ������ˮ�У�û�仯����ʯ����ˮ��Ӧ�����������ƣ��ų������ȣ��������������ܣ�Һ�����ǣ���B��ȷ��
C����������ͭ��Һ��Ӧ�����Ȼ�������ͭ���������к�ɫ���ʲ���������ɫ��������Ϊ��ʯ�ң���C��ȷ����ѡABC��
��4������Ҫ��Fe3O490%�Ĵ�����ʯ��������x��

![]() x=2425t
x=2425t
����Ҫ��Fe3O492.8%�Ĵ������������2425t��

����Ŀ����ѧ���ڷ��ӡ�ԭ��ˮƽ���о����ʵ���ɡ��ṹ�����ʼ���仯���ɵ�һ�Ż�����Ȼ��ѧ���ش���������
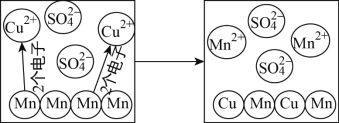
��1���ڶ�����̼�����������������������У�����������________���ɵģ�д�������ӵ����ƣ������ֶ�����̼��ѧ���ʵ���С������________��д�������ӵ����ƣ���
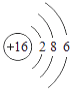
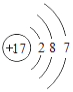
��2������г��˲���Ԫ�ص�ԭ�ӽṹʾ��ͼ���ش���������
O | Mg | S | Cl |
|
|
|
|
��ԭ���ڻ�ѧ��Ӧ������________�������õ�������ʧȥ�������ӣ���þԪ�غ���Ԫ����ɻ�����Ļ�ѧʽΪ________����Ԫ�غ���Ԫ�ػ�ѧ���ʾ��������Ե�ԭ��������ԭ�ӵ�________��ͬ��
��3�����ж������е������÷������֪ʶ���Ͳ���ȷ����________��
A���ڲ廨������Ʈ�㣬˵�����Ӳ��ϵ��˶�
Bˮ����ʱ��������ǣ�˵�����ӵĴ�С���¶����߶�����
C10mL�ƾ���10mLˮ��Ϻ����С��20mL��˵������֮���м��
Dʪ�·����ڻ�¯�ԣ��ɵýϿ죬˵�������˶��������¶����߶�����