��Ŀ����
�ٿ�ѧ�ҵ��㼣
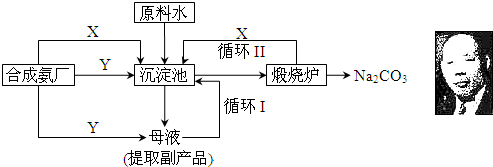
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4Cl���壬���ˣ��õ�NH4Cl��
�Իش��������⣺
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ ���ڹ�ҵ�����У�Ҫ������������ԭ��CO2д�����ʵ�õ���ȡ������̼�ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ���� ��
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ����� ��
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ���� ���ڢ��У��������ľ�����˺���Һ���������ٵ������� ����Һ����Ȼ���ڵ������� �����з�Ӧ�Ļ�ѧ����ʽΪ ��
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4Cl���壬���ˣ��õ�NH4Cl��
�Իش��������⣺
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ����
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ�����
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ����
��ϰ��ϵ�д�
�����Ŀ
����ѧУ��ͼ����Ż��ˣ����������ʲô������𣨡�����
| A������ĭ�������� | B���øɷ��������� | C���ö�����̼�������� | D����ˮ���� |
�Ӻ�ˮ����ȡ�Ĺ㷺���ڻ���������ͷɻ�����ҵ������Ϊ���������������ǣ�������
| A��Ca | B��Mg | C��K | D��Fe |
�ҹ���ѧ�Һ�°����������ġ������Ƽ�������̼�ͼ��ͼ������Ӧԭ��ΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3�����������ٽ��������Ƽ���ķ�չ������˵����ȷ���ǣ�������


| A���������еõ��ij�����NaHCO3����Ϊ��������NaHCO3���ܽ�ȱ�NH4ClС | B�����˵õ��ġ�ĸҺ����һ��ֻ������������ | C��ͼ��X�����ǰ��� | D��ͨ�백����������ʹ��Һ�ʼ��ԣ��ٽ�������̼�����գ�������������� |
��NaCl+CO2+NH3+H2O�TNaHCO3+NaCl���ǰ���ƴ����е���Ҫ��Ӧ�����������ʶ�У�����ȷ���ǣ�������
| A���ڼ�ѹ���������Ͱ���ˮ��ͨCO2���������ڷ�Ӧ�ķ��� | B��������������Һ��NaHCO3�ı�����Һ | C����������NaHCO3���ܽ�ȱ�NH4Cl�Ĵ�����NaHCO3�Ƚᾧ���� | D�����������ʣ����Һ�к����������� |
̼��һ����̼�������ֱ����������ͭ��Ӧ������˵����ȷ���ǣ�������
| A��������Ӧ�������û���Ӧ | B����Ӧ��ͭԪ���ڹ������������������� | C�����ɲ�����ͬ��ʵ��װ�� | D����Ӧ������е���������������ͭ�е��� |