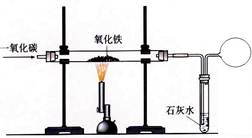
��Ŀ����
��ͼ��ʾ����ֻU���зֱ���봿���������ʵ��������� �����ۣ�ͬʱ������ע�����ڵ�Һ�壨��������Ũ��Ϊ20%�� ϡ���ᣩ����ע��U���С�
��д��������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽ ��16�� ��
��Ӧ��������ȴ�����£���������������ͳ̶����Դ����Ҷˣ�������һֻU���ڵ�������ʣ�࣬ ���� ��17�� ������ҡ����˵�U�ܡ�
���������ۺ�ϡ���ỻ���������ֻ����������Һ����Ҳ�ܹ۲쵽�����������ͣ����������ʿ����ǣ�д��ѧʽ�� ��18�� ��
��16�� Fe+ H2SO4��H2��+ FeSO4;��17����;��18��Na2CO3 HCl
����

��ϰ��ϵ�д�
�����Ŀ
��7�֣�ʹ���ܶ�С��ǿ�ȴ��þ�Ͻ��ܼ����������أ��Ӷ������������ĺͷ����ŷš�
|
��3����þ��ԭ��MgO�ɴӺ�ˮ�л�á�С������þ�����ᷴӦ��ķ�Һ��ģ��Ӻ�ˮ�л�ȡMgO�Ĺ��̣�ʵ�����£�
����1����������Һ�У��߽���߷�������CaO����MgCl2��ȫ����Ϊֹ�����˵�Mg(OH)2���塣������8.4g CaO��
����2����Mg(OH)2������ȷֽ�ΪMgO��ˮ������MgO������Ϊ4.0g ��
�ٲ���2��Mg(OH)2�ֽ�Ļ�ѧ����ʽΪ ��
��ͨ������MgO����������������Һ�к�MgCl2������m= g��
�۷���ʵ�����ݣ���֪������з����Ļ�ѧ��Ӧ�У�
CaO+H2O==Ca(OH)2��Ca(OH)2+MgCl2==CaCl2+Mg(OH)2���� ��
��2�֣���ͬ�����ʣ�����ɡ��ṹ���������ʵȷ��棬�������������������ԡ�������һ�㣬���������Ǹ��õ�ѧ�û�ѧ���������
| �� �� | ������ | ������ |
| Fe��Cu��Mg | ���ܺ�AgNO3��Һ��Ӧ | |
| HCl��H2SO4��HNO3 | | HCl�в�����Ԫ�� |
| C��CO��H2 | | CO�ǻ���������ǵ��� |
| CuSO4��MgSO4��ZnSO4 | ��������ˮ | |