��Ŀ����
��2013?�����ģ�⣩��ѧ����������ѧϰ��ѧ����Ҫ���ߣ���ͼΪ�������ӵĽṹʾ��ͼ���밴Ҫ����գ�

��1������ͬ��Ԫ�ص���
��2����������ȶ��ṹ��ԭ����
��3����F�Ļ�ѧ�������Ƶ�������
��4��ijԪ��R�γɻ�����Ļ�ѧʽ��R2SO4����û������ж�Ӧ��R��ԭ�ӽṹʾ��ͼ������
��5����A��C��FԪ�ع�ͬ��ɵ�һ�ּ���׳���
��6��д��ʵ������A��C��Ԫ����ɵĻ�������ȡ�����Ļ�ѧ����ʽ

��1������ͬ��Ԫ�ص���
DF
DF
������ţ�����2����������ȶ��ṹ��ԭ����
He
He
�������ӷ��ţ�����3����F�Ļ�ѧ�������Ƶ�������
G
G
������ţ�����4��ijԪ��R�γɻ�����Ļ�ѧʽ��R2SO4����û������ж�Ӧ��R��ԭ�ӽṹʾ��ͼ������
AFG
AFG
������ţ����û���������Ԫ�صĻ��ϼ�Ϊ+6
+6
����5����A��C��FԪ�ع�ͬ��ɵ�һ�ּ���׳���
�ռ�
�ռ�
������һ�֣�����6��д��ʵ������A��C��Ԫ����ɵĻ�������ȡ�����Ļ�ѧ����ʽ
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��������1������Ԫ�صĶ�������жϣ�
��2����������ȶ��ṹ�ĺ��弰���ӵĽṹʾ��ͼ������
��3�����������ĵ�������Ҫ����Ԫ�ص����ʷ�����
��4�����ݻ��ϼ�ԭ����R2SO4��R�Ļ��ϼۣ���������R��ԭ�ӽṹʾ��ͼ������������Ļ��ϼ����S�Ļ��ϼۣ�
��5������Ԫ��������ʣ�д��������
��6������Ԫ��������ʣ�д����Ӧ�ķ���ʽ��
��2����������ȶ��ṹ�ĺ��弰���ӵĽṹʾ��ͼ������
��3�����������ĵ�������Ҫ����Ԫ�ص����ʷ�����
��4�����ݻ��ϼ�ԭ����R2SO4��R�Ļ��ϼۣ���������R��ԭ�ӽṹʾ��ͼ������������Ļ��ϼ����S�Ļ��ϼۣ�
��5������Ԫ��������ʣ�д��������
��6������Ԫ��������ʣ�д����Ӧ�ķ���ʽ��
����⣺��1����Ԫ�صĶ����֪��DF�ĺ�����������ͬ������ͬһ��Ԫ�أ�
��2�������ӵĽṹʾ��ͼ��֪��B����ֻ��һ�����Ӳ㣬�ò�����2�����ӣ������ȶ��ṹ�������Ǻ�ԭ�ӣ�����ΪHe��
��3�������ӵĽṹʾ��ͼ��֪������FG��������������ͬ����1���������ƵĻ�ѧ���ʣ�
��4������������Ļ��ϼ�Ϊ-2�ۣ���R�Ļ��ϼ�Ϊ+1�ۣ����жϳ�Ԫ��R��ԭ���ڻ�ѧ�仯����ʧ1�����ӣ���AFG�����������1������С��4����ѧ�仯����ʧ1�����ӣ���ˣ�R��ԭ�ӽṹʾ��ͼ������AFG����������-2�ۣ��ڸû���������Ԫ�صĻ��ϼ�Ϊ+6��
��5�������ӵĽṹʾ��ͼ��֪��A��C��FԪ�طֱ����⡢������Ԫ�أ�����ɵļ����������ƣ��������ռ�������ƣ�
��6��������������֪��A��C��Ԫ����ɵĻ������ǹ������������ȡ��������Ӧ�Ļ�ѧ����ʽ�ǣ�2H2O2
2H2O+O2����
�ʴ�Ϊ����1��DF����2��He����3��G����4��AFG��+6����5���ռ���������ƣ�����6��2H2O2
2H2O+O2����
��2�������ӵĽṹʾ��ͼ��֪��B����ֻ��һ�����Ӳ㣬�ò�����2�����ӣ������ȶ��ṹ�������Ǻ�ԭ�ӣ�����ΪHe��
��3�������ӵĽṹʾ��ͼ��֪������FG��������������ͬ����1���������ƵĻ�ѧ���ʣ�
��4������������Ļ��ϼ�Ϊ-2�ۣ���R�Ļ��ϼ�Ϊ+1�ۣ����жϳ�Ԫ��R��ԭ���ڻ�ѧ�仯����ʧ1�����ӣ���AFG�����������1������С��4����ѧ�仯����ʧ1�����ӣ���ˣ�R��ԭ�ӽṹʾ��ͼ������AFG����������-2�ۣ��ڸû���������Ԫ�صĻ��ϼ�Ϊ+6��
��5�������ӵĽṹʾ��ͼ��֪��A��C��FԪ�طֱ����⡢������Ԫ�أ�����ɵļ����������ƣ��������ռ�������ƣ�
��6��������������֪��A��C��Ԫ����ɵĻ������ǹ������������ȡ��������Ӧ�Ļ�ѧ����ʽ�ǣ�2H2O2
| ||
�ʴ�Ϊ����1��DF����2��He����3��G����4��AFG��+6����5���ռ���������ƣ�����6��2H2O2
| ||
������ֻ�������������ӽṹʾ��ͼ�����ӹ��ɡ����ʵĹ�ϵ������˳����������ӽṹʾ��ͼ��ص����⣮

��ϰ��ϵ�д�
�����Ŀ
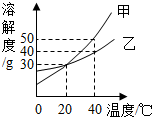 ��2013?�����ģ�⣩��ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵������ȷ���ǣ�������
��2013?�����ģ�⣩��ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵������ȷ���ǣ�������
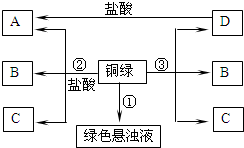 ��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��
��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��