��Ŀ����
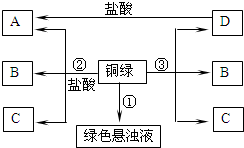 ��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��
��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��ˮ
ˮ
�����ʷ����������������ռ�һЩ�����ʣ��ͼ���ͬѧ����ͼ����������ʵ�飮����գ���1��ʵ�������ͭ���м�������������ܼ�B���ټ��������ڣ�������Ȼ����ɫ��ʵ��ڡ�����C��һ�ֳ������������壬C�Ļ�ѧʽΪ
CO2
CO2
��Ϊ����ʵ��۷�Ӧ����ļ��ȣ�Ӧ��ʹ�õĹؼ������������ƾ���
�ƾ���
����2����A����Һ5��9�ε���5mL10%�����������еõ���ɫ��״�������Լ��ȼ���ɺ�ɫ����D��д����ɫ��״����ת��ΪD�Ļ�ѧ����ʽ
Cu��OH��2
CuO+H2O
| ||
Cu��OH��2
CuO+H2O
��
| ||
��3��д��ʵ��۷�Ӧ�Ļ�ѧ����ʽ
Cu2��OH��2CO3
2CuO+H2O+CO2��
| ||
Cu2��OH��2CO3
2CuO+H2O+CO2��
���ڴ˹����У��ж�ͭ��û����ȫ��Ӧ��������
| ||
��ɫ����û����ȫ��ʧ
��ɫ����û����ȫ��ʧ
����4����֪Ca��HCO3��2
| ||
CuO��Cu2��OH��2CO3��Cu��OH��2
CuO��Cu2��OH��2CO3��Cu��OH��2
����д��ѧʽ������5���������ۣ�ͬѧ�ǽ���Сͮ������
ϡ����
ϡ����
��ȥ�����ϵ���ɫ���ʣ��������������е�֪ʶ���з�����ͭ����������ˮ�Ͷ�����̼����ʱ�����⣬������̼���������ЧӦ����Ҫ���壬����ʹ�õ������Ǿƾ��ƣ�������ͭ����ɫ��״�������ݴ˽�ɣ�
����⣺ͭ����������ˮ�Ͷ�����̼����ʱ�����⣬���ˮ��
ͭ�������ֽܷ���������ͭ��ˮ�Ͷ�����̼��C���γ�����ЧӦ����Ҫ���壬��C�Ƕ�����̼��D�Ǻ�ɫ���ʣ���D������ͭ����B��ˮ������ͭ�������ᷴӦ�����Ȼ�ͭ��ˮ����A���Ȼ�ͭ��
��1��C��һ�ֳ������������壬��C�Ƕ�����̼������ʱʹ�õ������Ǿƾ��ƣ����CO2���ƾ��ƣ�
��2��A���Ȼ�ͭ����������������Һ��Ӧ����������ͭ��ɫ��״������������ͭ�����ֽܷ���������ͭ��ˮ�����Cu��OH��2
CuO+H2O��
��3��ͭ�������ֽܷ���������ͭ��ˮ�Ͷ�����̼����û����ȫ�ֽ⣬����۲쵽��ɫ����û����ȫ��ʧ�����Cu2��OH��2CO3
2CuO+H2O+CO2������ɫ����û����ȫ��ʧ��
��4����ʶ̼��ͭ��������ͭ���ֽܷ���������ͭ��˵������ͭ�����ȶ�����ǿ��������ͭ�Լ��ȼ��ֽ���������ͭ��˵��������ͭ�����ȶ����������ʣ����CuO��Cu2��OH��2CO3��Cu��OH��2��
��5��ͭ���������ᷴӦ���ʿ���ʹ�������ȥ�����ϵ���ɫ���ʣ����ϡ���ᣮ
ͭ�������ֽܷ���������ͭ��ˮ�Ͷ�����̼��C���γ�����ЧӦ����Ҫ���壬��C�Ƕ�����̼��D�Ǻ�ɫ���ʣ���D������ͭ����B��ˮ������ͭ�������ᷴӦ�����Ȼ�ͭ��ˮ����A���Ȼ�ͭ��
��1��C��һ�ֳ������������壬��C�Ƕ�����̼������ʱʹ�õ������Ǿƾ��ƣ����CO2���ƾ��ƣ�
��2��A���Ȼ�ͭ����������������Һ��Ӧ����������ͭ��ɫ��״������������ͭ�����ֽܷ���������ͭ��ˮ�����Cu��OH��2
| ||
��3��ͭ�������ֽܷ���������ͭ��ˮ�Ͷ�����̼����û����ȫ�ֽ⣬����۲쵽��ɫ����û����ȫ��ʧ�����Cu2��OH��2CO3
| ||
��4����ʶ̼��ͭ��������ͭ���ֽܷ���������ͭ��˵������ͭ�����ȶ�����ǿ��������ͭ�Լ��ȼ��ֽ���������ͭ��˵��������ͭ�����ȶ����������ʣ����CuO��Cu2��OH��2CO3��Cu��OH��2��
��5��ͭ���������ᷴӦ���ʿ���ʹ�������ȥ�����ϵ���ɫ���ʣ����ϡ���ᣮ
���������⿼���˳������ʳɷֵ��ƶϣ���ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
�����Ŀ
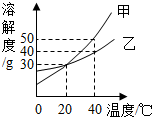 ��2013?�����ģ�⣩��ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵������ȷ���ǣ�������
��2013?�����ģ�⣩��ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵������ȷ���ǣ�������