��Ŀ����
 ��2010?�������ʼ죩2010��3��22�ա�����ˮ�ա�������Ļ���⣺���������ˮԴ�����콡�����硱���ҹ��������ˮ�ա��Ϳ�չ���й�ˮ�ܡ������������Ϊ���ϸ�ˮ��Դ���������Ͽɳ�����չ����
��2010?�������ʼ죩2010��3��22�ա�����ˮ�ա�������Ļ���⣺���������ˮԴ�����콡�����硱���ҹ��������ˮ�ա��Ϳ�չ���й�ˮ�ܡ������������Ϊ���ϸ�ˮ��Դ���������Ͽɳ�����չ������1����עˮ��Դ������������ͬ����Ҫ���ⶨˮ����pH��ѡ��
B
B
�������ţ�A����̪��Һ B��pH��ֽ C��ʯ����Һ
��2������ˮ����Ӳˮ������ˮ�����õ�������
����ˮ
����ˮ
����3�������ҹ�����5ʡ���ֵ����������ظɺ�����ǧ������ˮ���ѣ������ϧˮ����Լˮ������ˮ���������Ľ��飺
Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ��
Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ��
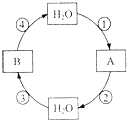
����4��ͼͼ����ˮΪ����ġ���ѧ��Ӧ��������������ǰһ����Ӧ��һ����������Ϊ��һ����Ӧ�ķ�Ӧ�������ź�Ҫ��Ļ�����Ӧ����ѭ��������ʾ��������з�Ӧ�Ļ�ѧ����ʽ��
ʾ�����ٷֽⷴӦ��2H2O
| ||
�ڻ��Ϸ�Ӧ��
2H2+O2
2H2O
| ||
2H2+O2
2H2O
��
| ||
�ܸ��ֽⷴӦ��
Ca��OH��2+2HCl�TCaCl2+2H2O
Ca��OH��2+2HCl�TCaCl2+2H2O
����������1����pH��ֽ���Բⶨ��Һ�����ȣ�
��2���÷���ˮ��������Ӳˮ����ˮ��
��3����Լ��ˮ��ÿ������Ӧ�������Σ�
��4����д��ѧ����ʽҪע��淶�ԣ�
��2���÷���ˮ��������Ӳˮ����ˮ��
��3����Լ��ˮ��ÿ������Ӧ�������Σ�
��4����д��ѧ����ʽҪע��淶�ԣ�
����⣺��1���ⶨˮ����pH��ѡ��pH��ֽ��
���B��
��2����ˮ�м������ˮʱ�������������ĭ�϶࣬����ˮ�������������ĭ���ٻ�����ĭ����Ӳˮ��
�������ˮ��
��3��Ҫ��ϧˮ����Լˮ������ˮ��Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ�ȣ�
���Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ�ȣ�
��4��������ȼ������ˮ�����ڻ��Ϸ�Ӧ����ѧ����ʽΪ��2H2+O2
2H2O��
���2H2+O2
2H2O��
��ˮ�������Ʒ�Ӧ�������������ƣ��������ƺ����ᷴӦ�������Ȼ��ƺ�ˮ�����ڸ��ֽⷴӦ����ѧ����ʽΪ��Ca��OH��2+2HCl�TCaCl2+2H2O��
���Ca��OH��2+2HCl�TCaCl2+2H2O��
���B��
��2����ˮ�м������ˮʱ�������������ĭ�϶࣬����ˮ�������������ĭ���ٻ�����ĭ����Ӳˮ��
�������ˮ��
��3��Ҫ��ϧˮ����Լˮ������ˮ��Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ�ȣ�
���Ҫ��Լÿһ��ˮ��������ˮ�����������������ˮ���ŷ���ˮ�ȣ�
��4��������ȼ������ˮ�����ڻ��Ϸ�Ӧ����ѧ����ʽΪ��2H2+O2
| ||
���2H2+O2
| ||
��ˮ�������Ʒ�Ӧ�������������ƣ��������ƺ����ᷴӦ�������Ȼ��ƺ�ˮ�����ڸ��ֽⷴӦ����ѧ����ʽΪ��Ca��OH��2+2HCl�TCaCl2+2H2O��
���Ca��OH��2+2HCl�TCaCl2+2H2O��
������ˮ��Դ�����˵���ѷ��ģ�Ҫ��Լ��ˮ��������Լ��ˮ������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ