��Ŀ����
����Ŀ����ͼ��ʾA��F�dz��л�ѧ���������ʡ�ͼ�С�������ʾת����ϵ����������ʾ��ܷ�Ӧ(�������ʺͷ�Ӧ����δ���)������A������θҺ�к��е��ᣬA��B�����кͷ�Ӧ�ɵõ������г��õ�һ�ֵ�ζƷ������C�����ڸ�������������
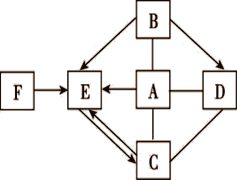
��1��A �Ļ�ѧʽΪ__________��B����Һ�д��ڵ�������____________��
��2�����ó��ڷ��õ�B�����ڿ�������ˮ���⣬�˹�����__________�����������ѧ�����仯��
��3��E��F�����������ͬ����E��F�ܷ�Ӧ���ȣ�FҲ����A��Һ������Ӧ����F��A��Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��4��C��D��Ӧ�ɵõ�B����C��D��Ӧ�Ļ�ѧ����ʽΪ______________________��
���𰸡� HCl ���������� ���� CaO + 2HCl = CaCl2 + H2O Na2CO3+Ca(OH)2=CaCO3��+2NaOH
����������1��A������θҺ�к��е��ᣬ��A�����ᣬʳ���������г��õ�һ�ֵ�ζƷ����Ҫ�ɷ����Ȼ��ƣ���B���������ƣ����������е������������������ӡ���2���������Ʒ�������ʱ�������������ɣ����������仯����3��E��F�����������ͬ����E��F�ܷ�Ӧ���ȣ���E��ˮ��F�������ƣ������������ᷴӦ�Ļ�ѧ����ʽ��CaO + 2HCl = CaCl2 + H2O ��4������C�����ڸ���������������C���������ƣ���ͼ��ת����ϵ��D��̼���ƣ�����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽΪNa2CO3+Ca(OH)2=CaCO3��+2NaOH

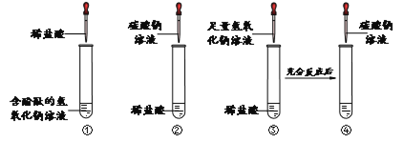
����Ŀ��������ͼ��ʾװ�ý���ʵ�飨ʵ��ǰK1��K2���رգ���

ʵ����װ���ڵ�ҩƷ���±���ʾ��
ע���� | A | B | |
ʵ��1 | ���� | NaHCO3��Һ | ����ʯ��ˮ |
ʵ��2 | NaOH��Һ | CO2���� | ��û����ˮ�еİ��ף��������п�����Ƭ�ϣ� |
��1��ʵ��1�д�K1������������ע��Aƿ�У��۲쵽Bƿ�г���ʯ��ˮ����ǣ�Bƿ�з�Ӧ�Ļ�ѧ����ʽΪ________����K2���ر�K1���۲쵽������Ϊ______��
��2��ʵ��2�У��۲쵽����ȼ�գ���ʵ�����Ϊ________��ͨ����ʵ��̽����ȼ��ȼ�������� ______��