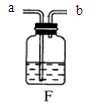��Ŀ����
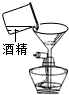
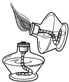
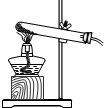
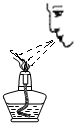
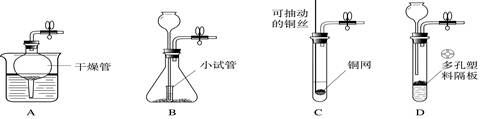
(10��) ����ͼ( I )�Dz��ֳ��û�ѧ������װ�ã�ͼ(��) ij��ʵ���װ��ͼ���Ա�ͼ(I)ͼ(��)�ش��������⣺
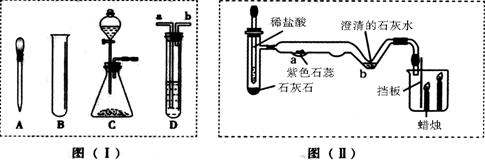
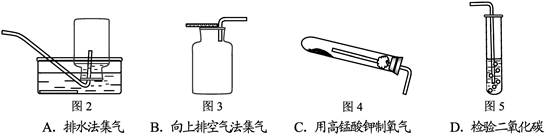
��1��ͼ( I )����C�п��Կ���Һ��μ��ٶȵ����������� ��
��2������Cװ����ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ ������Dװ�ø���H2 ����װ����ʢ�ŵ��Լ�Ϊ ������Ӧ�� ���ܿ�ͨ��(����ĸ��a����b��)��
��3����ͼ(��)�н�����ʵ�飬�Թ��еķ�Ӧ������a���������� ��b���ķ�Ӧ����ʽ�� ���ձ��е������� ��������˵�� ��
��4����ͬѧ��Ϊ��ͼ(��)ʵ��ʱ��b����һ����룬ԭ������� ��
��5������Ϊ����ʵ��װ�õ��ŵ��� (��һ�㼴��)��

��1��ͼ( I )����C�п��Կ���Һ��μ��ٶȵ����������� ��
��2������Cװ����ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ ������Dװ�ø���H2 ����װ����ʢ�ŵ��Լ�Ϊ ������Ӧ�� ���ܿ�ͨ��(����ĸ��a����b��)��
��3����ͼ(��)�н�����ʵ�飬�Թ��еķ�Ӧ������a���������� ��b���ķ�Ӧ����ʽ�� ���ձ��е������� ��������˵�� ��
��4����ͬѧ��Ϊ��ͼ(��)ʵ��ʱ��b����һ����룬ԭ������� ��
��5������Ϊ����ʵ��װ�õ��ŵ��� (��һ�㼴��)��
��1����Һ©������2��Zn+ 2HCl ZnCl2 + H2����Ũ���a����3�����ɫ��Ca(OH)2 + CO2 CaCO3��+ H2O ���ʹ�������Ϩ�𣨸ߴ������Ϩ�𣩣�CO2�ܶȱȿ������Ҳ�֧��ȼ�գ�����ȼ������4��CO2�к���HCl�������лӷ���HCl��ʯ��ˮ���ʣ���5����ʡҩƷ�����ڷ���ʵ��
�����������1����Һ©�����Կ���Һ��μ��ٶȣ���2����ȡ������Ӧ�Ļ�ѧ����ʽΪZn+2HCl=ZnCl2+H2����Ũ���������ˮ�Կ��Ը���H2������Ӧ��a���ܿ�ͨ�룻��3��������̼������ɫ��ʯ����Һ���ɫ������ʯ��ˮ�����ʷ�Ӧ����̼��Ƴ�����ˮ��CO2�ܶȱȿ�����֧��ȼ��Ҳ��ȼ�տ���ʹ�ձ���ȼ�յ�����������������Ϩ�𣻣�4��������лӷ��ԣ����CO2�к���HCl�������лӷ���HCl��ʯ��ˮ���ʣ�b����һ����룻��5����ʵ��װ�þ��н�ʡҩƷ�����ڷ���ʵ����ص㡣

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ