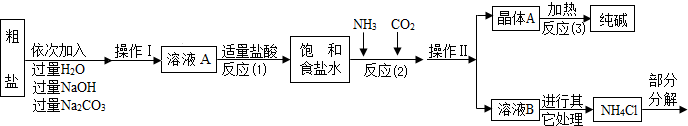
��Ŀ����
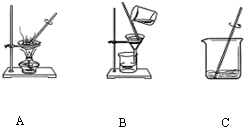
��1�������١��ڡ�������Ϊ��ͼ�еģ�����ĸ��
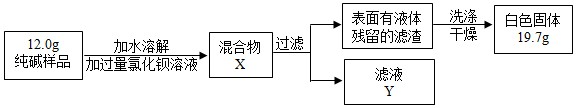
��2����ʵ����������NaCl����ƫ�٣�����ܵ�ʵ��ԭ����-������ĸ����
A������ʱ��ֽ������ B������ʱ�й��彦�� C���ܽ⺬����ɳ�Ĵ�ʳ��ʱ�������ˮ������
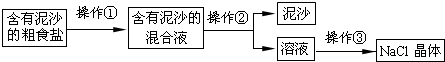
��3���ڵõ�ʳ�ι���Ĺ����У���
��4���������ϲ����õ���ʳ������100g 10%���Ȼ�����Һʱ���ش��������⣺
�ټ��㣺��Ҫ
�ڳ���������ʱ���������Ϊ23.1g���ձ�ʢ�Ź��壬��������ƽ���ѷ�30g�����룬����ƽ�ϵ�����λ��Ӧ����ͼ��
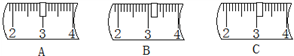
���ܽ⣺�ò�������Ҫ�õ����������е�a��e��
a���ձ���b��10mL��Ͳ��c��100mL��Ͳ��d���ιܡ�e����������f��ҩ�ס�g��������
������ˮʱ���Ӷ�������������Һ���Ȼ��Ƶ���������
��2����������ʱ�й��彦�����ܽ⺬����ɳ�Ĵ�ʳ��ʱ�������ˮ�����㶼�п���ʹ��������NaCl����ƫ�ٽ��н��
��3����������ʱ�õ�ʳ�ι���Ĺ����У����϶�������ʱ����Ҫֹͣ���Ƚ��н��
��4��������Һ���ƵIJ����Լ�ע��������н��
��2������ʱ�й��彦�����ܽ⺬����ɳ�Ĵ�ʳ��ʱ�������ˮ�����㶼�п���ʹ��������NaCl����ƫ�٣���ѡ��BC��
��3������ʱ�õ�ʳ�ι���Ĺ����У����϶�������ʱ����Ҫֹͣ���ȣ���������������Ƚ�ʣ��ˮ�����ɣ�����϶���壻
��4���ټ��㣺��Ҫ�Ȼ��ƹ��������=100g��10%=10g�����10��
�ڳ���������ʱ���������Ϊ23.1g���ձ�ʢ�Ź��壬���ձ����Ȼ��Ƶ�������=10g+23.1g=33.1g��������������ƽ���ѷ�30g�����룬���������Ϊ3.1g��������ƽ�ϵ�����λ��Ӧ����ͼB��ʾ�����B��
���ܽ⣺�ò�������Ҫ�õ������������ձ���100mL��Ͳ�������������c��
������ˮʱ���Ӷ�������ȡˮ��ʵ�����ҪС��90mL��������������Һ���Ȼ��Ƶ�������������10%��������ڣ�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д���ȤС��ι�ij�Ƽ���������Ϣ���������������о���
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl NH3��+HCl����
NH3��+HCl����
����ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��
���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ______��
�ڲ����������Ϊ______��
�۷�Ӧ��1���м����������������______��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳����______������ĸ����
A����ͨ�������̼��ͨ�������� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ���______������ĸ����
A��CO2��������B��NH3��������C��HCl�������� D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ______��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���
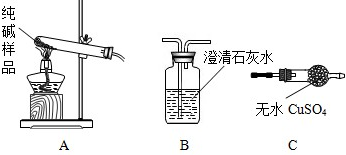
| ѡ���װ�� | ʵ������ | ʵ����� |
| ______ | ______ | ��Ʒ��������A |
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ______��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ�����______���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ�______���۲������жϣ�
A��BaCl2��Һ������ B��ϡH2SO4������C��Na2CO3��Һ����D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ______�� ��д��������̣�
��ȤС��ι�ij�Ƽ���������Ϣ���������������о���
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl NH3��+HCl����
NH3��+HCl����
����ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ��������
�ڲ����������Ϊ����
�۷�Ӧ��1���м��������������������
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳��������������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ�������������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ��������
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ���� | �� | ��Ʒ��������A |
�����̽������
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ����ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ������������۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ��������۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ�� ��д��������̣�
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl
 NH3��+HCl����
NH3��+HCl��������ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ ��
�ڲ����������Ϊ ��
�۷�Ӧ��1���м���������������� ��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳���� ������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ��� ������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ����� ���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ� ���۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̣�
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl
 NH3��+HCl����
NH3��+HCl��������ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ ��
�ڲ����������Ϊ ��
�۷�Ӧ��1���м���������������� ��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳���� ������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ��� ������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ����� ���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ� ���۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̣�