��Ŀ����
��7�֣�С��ͬѧΪ�ⶨijϡ������ҺŨ�ȣ���������������������ȡ��ϡ������Һ20 g����30g����������Һ�����μ����ϡ������Һ�У�������η�Ӧ���й����ݼ��±���
������й����ݽ��з�������㣺
��1��20g��ϡ������Һ������������Һ��ȫ��Ӧʱ���ɳ���������Ϊ g��
��2���������η�Ӧ�����õĻ��Һ���ˣ��õ���Һ������Ϊ g��
��3����ϡ������Һ��Ũ�ȣ�����������������Ϊ���٣���д��������̣�
��4��С��ͬʱ��pH�Ʋⶨ����¼��ϡ������Һ������������Һ��Ӧ�����л����ҺpH�仯���������ͼ��ʾ����������������ͼ�б����ϡ������Һ������������Һ�պ���ȫ��Ӧʱ�ı仯�㣬��ע��pH������Ӧ������������Һ��������
| | ��һ�� | �ڶ��� | ������ |
| ��������������Һ������/g | 10 | 10 | 10 |
| ���ɳ���������/g | 0.923 | 1.864 | 2.33 |
��1��20g��ϡ������Һ������������Һ��ȫ��Ӧʱ���ɳ���������Ϊ g��
��2���������η�Ӧ�����õĻ��Һ���ˣ��õ���Һ������Ϊ g��
��3����ϡ������Һ��Ũ�ȣ�����������������Ϊ���٣���д��������̣�
��4��С��ͬʱ��pH�Ʋⶨ����¼��ϡ������Һ������������Һ��Ӧ�����л����ҺpH�仯���������ͼ��ʾ����������������ͼ�б����ϡ������Һ������������Һ�պ���ȫ��Ӧʱ�ı仯�㣬��ע��pH������Ӧ������������Һ��������

��1��2.33 g��1�֣� ��2��47.67 g��1�֣�
��3��(��4�֣���δ֪��������͵�λ��0��5�֣���ѧ����ʽ0��5�֣�������ϵʽ1�֣�x���1�֣�������������1��)
�⣺20gϡ������Һ�к����������Ϊx
Ba(OH)2�� H2SO4�� BaSO4����2H2O
98 233
x 2.33 g
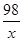 ��
�� x��0.98 g
x��0.98 g
��ϡ������Һ��Ũ�ȣ�����������������Ϊ="0.98" g��20 g��100%��4.9%
�𣺸�ϡ������Һ��Ũ�ȣ�����������������Ϊ4.9%��
��4����ͼ��1�֣�

��3��(��4�֣���δ֪��������͵�λ��0��5�֣���ѧ����ʽ0��5�֣�������ϵʽ1�֣�x���1�֣�������������1��)
�⣺20gϡ������Һ�к����������Ϊx
Ba(OH)2�� H2SO4�� BaSO4����2H2O
98 233
x 2.33 g
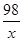 ��
�� x��0.98 g
x��0.98 g ��ϡ������Һ��Ũ�ȣ�����������������Ϊ="0.98" g��20 g��100%��4.9%
�𣺸�ϡ������Һ��Ũ�ȣ�����������������Ϊ4.9%��
��4����ͼ��1�֣�

��1��ͨ��ͼ�������֪����������������ﵽ30gʱ����Һ�ʼ��ԣ�������������ȫ��Ӧ����˿�֪���ɵ����ᱵ������Ϊ2.33g
��2�����ݵ����η�Ӧ�����õij����������Լ�������������������������غ㶨�����֪���˺�������Һ������Ϊ20g+30g-2.33g=47.67g
��3���⣺20gϡ������Һ�к����������Ϊx
Ba��OH��2+H2SO4=BaSO4��+2H2O
98 233
x 2.33 g
��ã�x="0.98" g
��ϡ������Һ��Ũ�ȣ�����������������Ϊ��0.98 g��20 g��100%��4.9%
��4������10g����������������Ӧ�ij�������Ϊ0.923g�����Կ�������2.33g������Ӧ������������Һ��������y������10g��0.923g= y��2.33g�����y��25g�ݴ˿ɻ���ͼ��
��2�����ݵ����η�Ӧ�����õij����������Լ�������������������������غ㶨�����֪���˺�������Һ������Ϊ20g+30g-2.33g=47.67g
��3���⣺20gϡ������Һ�к����������Ϊx
Ba��OH��2+H2SO4=BaSO4��+2H2O
98 233
x 2.33 g
��ã�x="0.98" g
��ϡ������Һ��Ũ�ȣ�����������������Ϊ��0.98 g��20 g��100%��4.9%
��4������10g����������������Ӧ�ij�������Ϊ0.923g�����Կ�������2.33g������Ӧ������������Һ��������y������10g��0.923g= y��2.33g�����y��25g�ݴ˿ɻ���ͼ��

��ϰ��ϵ�д�
�����Ŀ

 4Al + 3O2��������ӹ�һ��������Ҫ1.08 Kg������������Ҫ�����������������������Ƴ����������
4Al + 3O2��������ӹ�һ��������Ҫ1.08 Kg������������Ҫ�����������������������Ƴ����������