��Ŀ����
ˮ������������Դ��
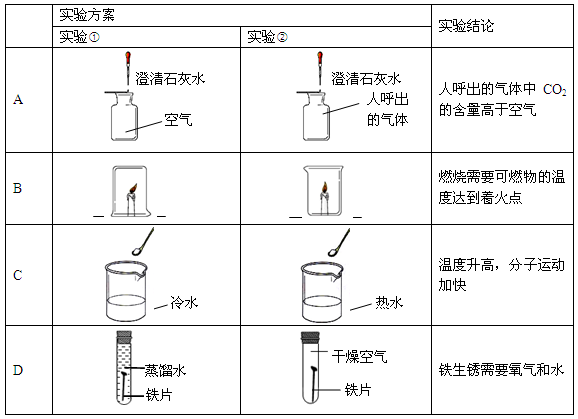
�ٳ�ȥˮ�����������ʵIJ�����______�����в�������������______��
��ˮ����������ʷ�����ѧ��Ӧ���Ծ�һ����д����ѧ����ʽ��______��
����ͼ�м��ǵ�����ˮ��ģ��װ�ã����ǵ��ˮ��װ�ã�
����ˮ��״̬�仯Ϊ��Һ̬����̬��Һ̬�����������ˮ���ӵ�______�����˸ı䣮����������ˮ����ȴ����������______
a��ˮ���ӵĵ���b�������ݴ�c����ɢ��
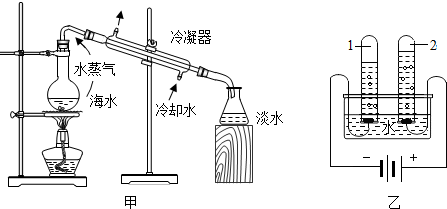
�������Թܡ�2��������ļ��鷽����______��ˮ�ֽ�����е���С����______������ĸ���� �ɱ�ʾһ��ˮ���ӣ�
�ɱ�ʾһ��ˮ���ӣ�
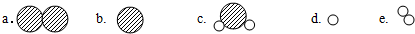
���϶����š�̽��ʹ�õ�Һ�⼴Ϊ���ˮ���ã���ȡ3mol H2����ˮ�����ʵ���Ϊ______
�����ݷ���ʽ��ʽ���㣩
�ٳ�ȥˮ�����������ʵIJ�����______�����в�������������______��
��ˮ����������ʷ�����ѧ��Ӧ���Ծ�һ����д����ѧ����ʽ��______��
����ͼ�м��ǵ�����ˮ��ģ��װ�ã����ǵ��ˮ��װ�ã�

����ˮ��״̬�仯Ϊ��Һ̬����̬��Һ̬�����������ˮ���ӵ�______�����˸ı䣮����������ˮ����ȴ����������______
a��ˮ���ӵĵ���b�������ݴ�c����ɢ��
�������Թܡ�2��������ļ��鷽����______��ˮ�ֽ�����е���С����______������ĸ����
 �ɱ�ʾһ��ˮ���ӣ�
�ɱ�ʾһ��ˮ���ӣ�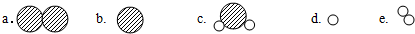
���϶����š�̽��ʹ�õ�Һ�⼴Ϊ���ˮ���ã���ȡ3mol H2����ˮ�����ʵ���Ϊ______
�����ݷ���ʽ��ʽ���㣩
�ٳ�ȥˮ�����������ʵIJ����ǹ��ˣ����в�������������������������ˣ�������
��ˮ����������ʷ�����ѧ��Ӧ������ˮ�������Ʒ�Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2�����CaO+H2O=Ca��OH��2��
�ۢ�ˮ����̬�仯������ˮ���ӵļ�������˸ı䣬����������ˮ����ȴ����������ˮ�ı����ݴ�����ʣ���������b��
��ˮͨ��ֽ��������������������������������������Ϊ2��1�����������Թܡ�2������������������������鷽���ǽ������ǵ�ľ�������Թ��ڣ���ľ����ȼ֤����������ˮ�ֽ�����е���С������ԭ�Ӻ���ԭ�ӣ�����������ǵ�ľ�������Թ��ڣ���ľ����ȼ֤����������bd��
��������ˮ�����ʵ���Ϊx��
2H2O
2H2��+O2��
2 2
x 3mol
=
x=3mol
������ˮ�����ʵ���Ϊ3mol��
��ˮ����������ʷ�����ѧ��Ӧ������ˮ�������Ʒ�Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2�����CaO+H2O=Ca��OH��2��
�ۢ�ˮ����̬�仯������ˮ���ӵļ�������˸ı䣬����������ˮ����ȴ����������ˮ�ı����ݴ�����ʣ���������b��
��ˮͨ��ֽ��������������������������������������Ϊ2��1�����������Թܡ�2������������������������鷽���ǽ������ǵ�ľ�������Թ��ڣ���ľ����ȼ֤����������ˮ�ֽ�����е���С������ԭ�Ӻ���ԭ�ӣ�����������ǵ�ľ�������Թ��ڣ���ľ����ȼ֤����������bd��
��������ˮ�����ʵ���Ϊx��
2H2O
| ||
2 2
x 3mol
| 2 |
| x |
| 2 |
| 3mol |
x=3mol
������ˮ�����ʵ���Ϊ3mol��

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
 ȡ��̿��
ȡ��̿��