��Ŀ����
����Ŀ����6�֣�������120g��������Ϊ22.5%��NaCl��Һ���������£�
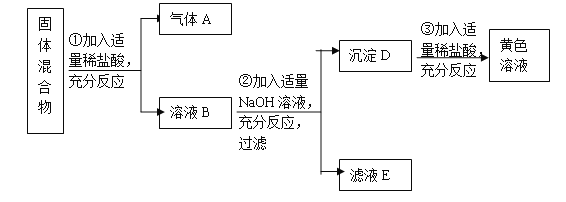
��1�����㣺��ҪNaCl������Ϊ________g��
��2������������ǰ��Ӧ�ȵ�����ƽ__________������Ͳ����ȡ�����ˮ��ˮ���ܶ�Ϊ1g/cm3��ʱ��Ӧѡ��_________��10mL��100 mL��200 mL������Ͳ��
��3���ܽ⣺�ܽ�ʱ���������������裬Ŀ����______________��
��4����˼����ȡ��Ͳ��ˮ�����ʱ�������Ӷ���������õ���Һ��NaCl����������_________���ƫ����ƫС������Ӱ�족��������NaClʱ������NaCl������λ���ߵ�������õ���Һ��NaCl����������_________���ƫ����ƫС������Ӱ�족����ע��1g���������룩��
��������1��27�� ��2����ƽƽ��������ƽ����Ⱥ���������100mL��
��3���ӿ��ܽ��� ��4��ƫС����Ӱ�졣
��������
�����������1����Ϊ���ʵ�����=��Һ����������Һ����������������������Ҫ�Ȼ��Ƶ�����=120g��22.5%=27g��
��2����ʹ��������ƽ����ǰ��Ҫ�ȵ�����ƽƽ�⣻��Ϊ��Ҫˮ��������120g-27g=93g��ˮ���ܶ���1g/mL������ˮ�������![]() =93mL����Ͳ������Ӧ�Դ���ˮ���������ѡ��100mL����Ͳ��
=93mL����Ͳ������Ӧ�Դ���ˮ���������ѡ��100mL����Ͳ��
��3���ܽ�ʱ���ò����������Ŀ���Ǽӿ��Ȼ��Ƶ��ܽ⣻
��4����ȡ��Ͳ��ˮ�����ʱ�������Ӷ���������ȡ��ˮ�����ƫ��ʹ��õ���Һ��NaCl��������ƫС������NaClʱ������NaCl������λ���ߵ�����Ϊ1g���������������Գ�ȡ27gʱ����Ҫ�����룬����ȡ���Ȼ��Ƶ���������27g�����Զ���õ���Һ��NaCl����������Ӱ�졣

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ��ij�ܱ���������X�������Ͷ�����̼�������ʣ���һ�������³�ַ�Ӧ,��÷�Ӧǰ������ʵ��������±������ݱ�����Ϣ���ж�����˵����ȷ����
���� | X | O2 | CO2 | H2O |
��Ӧǰ����/g | 16 | 70 | 1 | 0 |
��Ӧ������/g | 0 | ���� | 45 | 36 |
A���÷�ӦΪ�û���Ӧ
B��X��һ������̼��������Ԫ��
C�����С����⡱ֵΪ5
D����Ӧ���ɵ�CO2��H2O��������Ϊ45:36