��Ŀ����
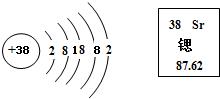
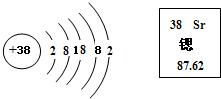
����ͬѧ�Բ�ͬ�Ļ�ѧ˼ά��ʽ���߹��ɸ����л�ѧ��Ӧ�����ͽ��з��࣬����Ϊ�� �������һ����:��CaO+H2O�TCa��OH��2����Mg��OH��2��MgO+H2O����Ba��OH��2+2HCl�TBaCl2+2H2O����Zn+H2SO4�TZnSO4+H2������2Mg+O2��ȼ2MgO��
�������һ����:��CaO+H2O�TCa��OH��2����Mg��OH��2��MgO+H2O����Ba��OH��2+2HCl�TBaCl2+2H2O����Zn+H2SO4�TZnSO4+H2������2Mg+O2��ȼ2MgO��
A. ���ڻ��Ϸ�Ӧ���Ǣ٢� B. ���ڸ��ֽⷴӦ���Ǣۢ�
C. �����������ų��ķ�Ӧ���Ǣڢ� D. ������Ԫ�ػ��ϼ۱仯���Ǣܢ�
D

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ