��Ŀ����
С���ڻ�ѧʵ���ҷ���ʢ������������Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ�����Ǿͽ���С����С�칲ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�����������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺
��1���ٿ�����NaOH �ڿ�����Na2CO3 �ۿ����� _______________ ��Ϊ����֤���룬���Ƿֱ���������ʵ�飺
��2��С��ȡ������ɫ��ĩ�������еμ�ϡ���ᣬ���������ɣ��ɴ�С����Ϊ��ɫ��ĩ��̼���ƣ�С�յĽ����Ƿ���ȷ�� _______ �������� _______________________________��
��3��С��ȡ������ɫ��ĩ�����Һ�������еμӷ�̪��Һ����Һ��Ϊ��ɫ���ɴ�С���жϰ�ɫ��ĩΪ�������ƣ�С���Ľ����Ƿ���ȷ�� _______�������� _______________________________ ��
��4��С��ȡ������ɫ��ĩ�����Һ�������еμ��Ȼ�����Һ���а�ɫ�����������ɴ�С���жϰ�ɫ��ĩ���� ___________��Ϊ����֤����ۣ�С���������Һ�еμ��Ȼ�����Һ�������ٲ���������С��������Ŀ����_______________________ ��Ȼ����ˣ�����Ϊ����������Ӧ���е�ʵ���� _____________________ ����С��������ʵ���У������Ȼ�����Һ��������������Һ�Ƿ���У� ______ �������� _________________________�����û�ѧ����ʽ��ʾ��
��2������ȷ��NaOH��Na2CO3 �Ļ�����������Ҳ�ܲ������ݣ�
��3������ȷ��̼������Һ�Լ��ԣ�Ҳ��ʹ��̪��Һ���ɫ��
��4��Na2CO3 ����ȥ������е�̼���ƣ�����Һ�м�����ɫ��̪��Һ�����У�Ba��OH��2+Na2CO3=BaCO3��+2NaOH��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�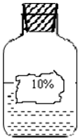 29��С���ڻ�ѧʵ���ҷ���һƿ��ɫ��Һ��ͼ����ʦ����������ƿ�������������ơ��Ȼ��ơ�̼���ơ�Ũ����������Һ�е�һ�֣�ʵ��ʱ���õ�ҩƷ�У�KNO3��BaCl2��ϡ����������Һ����
29��С���ڻ�ѧʵ���ҷ���һƿ��ɫ��Һ��ͼ����ʦ����������ƿ�������������ơ��Ȼ��ơ�̼���ơ�Ũ����������Һ�е�һ�֣�ʵ��ʱ���õ�ҩƷ�У�KNO3��BaCl2��ϡ����������Һ����