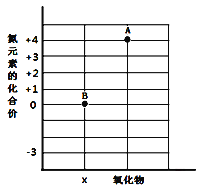
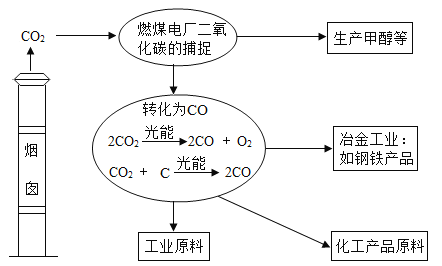
��Ŀ����
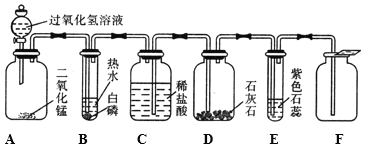
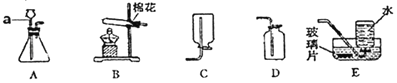
����Ŀ���ú��ж�����̼��ˮ�������ʵ�ij�ֻ�ԭ������ⶨһ������������(FexOy)����ɣ�ʵ��װ������ͼ��ʾ��������ͼ�Իش�
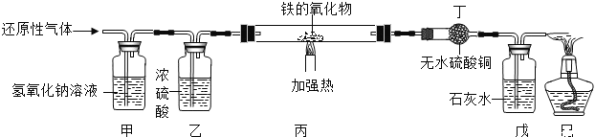
(1)��װ�õ�������_____����Ӧ�Ļ�ѧ����ʽ��_____��
(2)�������װ�ú���װ��λ�õ�˳���������ʵ������Ӱ�죿______��
(3)ʵ������ж�װ����û�����Ա仯������װ������Һ�����˰�ɫ��������û�ԭ��������____��
(4)����װ���е�FexOyȫ������ԭ����ʣ����������Ϊ16.8�ˣ�ͬʱ�����װ�õ�����������17.6�ˣ���FexOy�У���Ԫ�غ���Ԫ�ص�������Ϊ______��������������Ļ�ѧʽΪ____��
(5)����ʵ��װ���У����û�м�װ�ã���ʹ�ⶨ�������Ԫ������Ԫ�ص������ı�ֵ_____(��ƫ��ƫС����Ӱ��)�����û����װ�ã����ܲ����ĺ����_____��
���𰸡���ȥ������̼ 2NaOH+CO2�TNa2CO3+H2O ��Ӱ�� һ����̼ 21��8 Fe3O4 ƫС �ж���һ����̼����Ⱦ����
��������
��Ϊ�ۺ�ʵ�������û�ԭ������ⶨ�������������ɣ���ԭ���Ǹ��������������ͨ������ȷ��������ͳ��нΣ���ԭ��������������һ����̼�����Ƕ���������ˮ�Ͷ�����̼��ԭ�����л��ж�����̼��ˮ���������Ա����ȳ�ȥ��Ȼ���û�ԭ�����巴Ӧ������װ�����Լ������ɵ�ˮ�Ͷ�����̼�����ļ�װ�ý���β���������л���������
��1���������̼�����������Ʒ�Ӧ������ʽΪ��2NaOH+CO2�TNa2CO3+H2O�����Գ�������̼������������Һ���ʼ�װ�õ������dz�ȥ�������գ�������̼��
��2����Ũ���������ˮ�ԣ����Գ�ˮ����ѡ��Ũ��������������̼ʱ������Ҫ����Һ��ͨ���������ˮ����������Ҫ�ȳ�������̼�ٸ���������װ�ú���װ��λ�õ�˳���������ʵ�����Ӱ����
��3����װ��ʢ�ŵ�����ˮ����ͭ����ˮ����ͭ��ˮ���������װ��û�����Ա仯��˵��û��ˮ���ɣ��ʸû�ԭ�����岻����������װ��ʢ�ŵ���ʯ��ˮ��������̼��ʹʯ��ˮ����ǣ���װ������Һ�����˰�ɫ�������ʸû�ԭ��������һ����̼��
��4����װ���е�FexOyȫ������ԭ��ʣ�����Ϊ���ɵ�������������װ����ʢ�ŵ�ʯ��ˮ��ˮ������̼�����ӵ�����Ϊ������̼��������������̼��һ����̼��ȡFexOy�������ɵģ���������ֻ̼��һ����ԭ���������������ռ������̼������![]() ����FexOy����Ԫ�ص�����Ϊ��17.6g��
����FexOy����Ԫ�ص�����Ϊ��17.6g��![]() =6.4g����FexOy������Ԫ�ص�������Ϊ��56x��16y=16.8g��6.4g=21��8��x��y=3��4��������������Ļ�ѧʽΪ Fe3O4��
=6.4g����FexOy������Ԫ�ص�������Ϊ��56x��16y=16.8g��6.4g=21��8��x��y=3��4��������������Ļ�ѧʽΪ Fe3O4��
��4�����û�м�װ�ã����еĶ�����̼Ҳ������װ�ñ����գ��ʶ�����̼������ƫ���൱��һ����̼��õ������������ӣ��ʲ�ý������Ԫ������Ԫ�ص������ı�ֵƫС��һ����̼�ж�������Ⱦ���������ļ�װ�ö�β�����д��������л���������

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�