题目内容
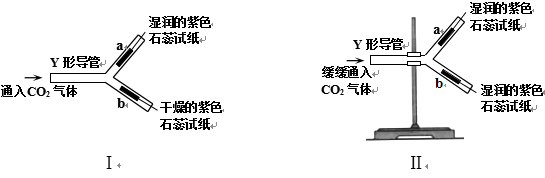
【题目】某兴趣小组为探究“氧化铁和铜粉”混合物中铜的质量分数,称取一定质量的混合物,按照下图实验装置进行实验:

Ⅰ 定性分析
(1)实验时要“先通一氧化碳气体,后加热”的目的是__________。
(2)装置A中发生反应的方程式为__________。
(3)实验装置B中的现象是___________,发生反应的化学方程式为___________。
(4)该装置的设计有一明显不当之处,你的改进方案是___________。
Ⅱ 定量分析
该兴趣小组按照科学的方案完成实验后,对通入足量CO充分反应后的管内固体X进行如下后续实验探究:
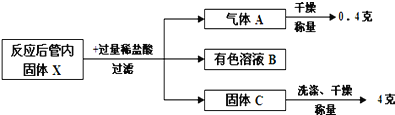
回答下列问题:
(5)写出固体X与稀盐酸反应的方程式__________。
(6)根据以上实验流程,计算原混合物“氧化铁和铜粉”中铜的质量分数为_________。
【答案】 排尽管内空气,防止发生爆炸 3CO+ Fe2O3高温2Fe+3CO2 澄清石灰水变浑浊 Ca(OH)2+CO2=CaCO3↓+H2O 在尾导管处放置一点燃的酒精灯 Fe+2HCl=FeCl2+H2↑ 20%
【解析】(1)一氧化碳具有可燃性,点燃时会可能发生爆炸,实验时要“先通一氧化碳气体,后加热”目的是排尽玻璃管内的空气,防止加热时发生爆炸;(2)一氧化碳还原氧化铁生成铁和二氧化碳,化学方程式为3CO+Fe2O3![]() 2Fe+3CO2(3)实验装置B中二氧化碳通入石灰水中,现象是 澄清石灰水变浑浊;发生反应的化学方程式CO2+Ca(OH)2═CaCO3↓+H2O;(4)一氧化碳有剧毒,不能直接排放到空气中,故在尾导管处放置一点燃的酒精灯;定量分析:(5)反应后管内固体是铁和铜的混合物,加入过量盐酸后,铁与盐酸反应产生氢气,化学方程式为Fe+2HCl═FeCl2+H2↑;(6)根据氢气的质量可求出铁的质量,根据铁元素的质量可求出氧化铁的质量,(6)设铁的质量为
2Fe+3CO2(3)实验装置B中二氧化碳通入石灰水中,现象是 澄清石灰水变浑浊;发生反应的化学方程式CO2+Ca(OH)2═CaCO3↓+H2O;(4)一氧化碳有剧毒,不能直接排放到空气中,故在尾导管处放置一点燃的酒精灯;定量分析:(5)反应后管内固体是铁和铜的混合物,加入过量盐酸后,铁与盐酸反应产生氢气,化学方程式为Fe+2HCl═FeCl2+H2↑;(6)根据氢气的质量可求出铁的质量,根据铁元素的质量可求出氧化铁的质量,(6)设铁的质量为![]() ,
,
Fe+2HCl═FeCl2+H2↑
56 2
![]() 0.4g
0.4g
![]()
![]() =11.2g
=11.2g
所以氧化铁的质量为![]() =16g,所以原混合物“氧化铁和铜粉”中铜的质量分数为:
=16g,所以原混合物“氧化铁和铜粉”中铜的质量分数为:
![]() 。
。

 鸿图图书寒假作业假期作业吉林大学出版社系列答案
鸿图图书寒假作业假期作业吉林大学出版社系列答案