��Ŀ����
ij�������ʵ�CaCO3���壨���ʲ�����ˮ��Ҳ�����ᷴӦ������ʦ����ͬѧ���ⶨ�ù���������CaCO3����������ʵ�飮���õ�����ƽ�ⶨ���й�ʵ���������±���| ������Ŀ | ������g�� |
| ���ʺ� CaCO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���ᣨ������ | 141.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��55�룩 | 149.00 |
��2����ͨ�������������������CaCO3������������______��
���𰸡���������1�����������غ㶨�ɼ����������̼��������
��2�����ݼ�����Ķ�����̼�����������÷���ʽ�еı�����ϵ���������̼��Ƶ��������ڼ���̼��Ƶ�����������
����⣺
��1����Ӧǰ����������151.2�ˣ���Ӧ�����������149�ˣ�����CO2����Ϊ��10g+141.2g-149.0g=2.2g
��2��������2.2gCO2��ҪCaCO3����Ϊx
CaCO3+2HCl�TCaC2l+H2O+CO2��
100 44
X 2.2g
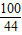 =
=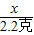 x=5g
x=5g
W��CaCO3��=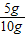 ×100%=50%��
×100%=50%��
�����ɶ�����̼2.2�ˣ���Ʒ��̼��Ƶ�����������50%��
�ʴ�Ϊ��2.2�ˣ�50%��
�������ڽ������ʱ�����ȷ������еķ�Ӧ��ԭ���Լ��������ݣ�Ȼ����ѧ����֪ʶ���з������
��2�����ݼ�����Ķ�����̼�����������÷���ʽ�еı�����ϵ���������̼��Ƶ��������ڼ���̼��Ƶ�����������
����⣺
��1����Ӧǰ����������151.2�ˣ���Ӧ�����������149�ˣ�����CO2����Ϊ��10g+141.2g-149.0g=2.2g
��2��������2.2gCO2��ҪCaCO3����Ϊx
CaCO3+2HCl�TCaC2l+H2O+CO2��
100 44
X 2.2g
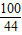 =
=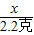 x=5g
x=5g W��CaCO3��=
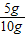 ×100%=50%��
×100%=50%�������ɶ�����̼2.2�ˣ���Ʒ��̼��Ƶ�����������50%��
�ʴ�Ϊ��2.2�ˣ�50%��
�������ڽ������ʱ�����ȷ������еķ�Ӧ��ԭ���Լ��������ݣ�Ȼ����ѧ����֪ʶ���з������

��ϰ��ϵ�д�
�����Ŀ
ij�������ʵ�CaCO3���壨���ʲ�����ˮ��Ҳ�����ᷴӦ������ʦ����ͬѧ���ⶨ�ù���������CaCO3����������ʵ�飮���õ�����ƽ�ⶨ���й�ʵ���������±���
��1�����ɶ�����̼����g��
��2����ͨ�������������������CaCO3������������ ��
| ������Ŀ | ������g�� |
| ���ʺ� CaCO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���ᣨ������ | 141.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��55�룩 | 149.00 |
��2����ͨ�������������������CaCO3������������
ij�������ʵ�CaCO3���壨���ʲ�����ˮ��Ҳ�����ᷴӦ������ʦ����ͬѧ���ⶨ�ù���������CaCO3����������ʵ�飮���õ�����ƽ�ⶨ���й�ʵ���������±���
| ������Ŀ | ������g�� |
| ���ʺ� CaCO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���ᣨ������ | 141.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ����+���ʺ�CaCO3�Ļ���� ����Ӧ��ʼ��55�룩 | 149.00 |
��2����ͨ�������������������CaCO3������������______��