��Ŀ����
Ϊ�ⶨijͭп�Ͻ���п������������ijͬѧ���úϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��������������/g | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������________ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ________��
�⣺п������ǡ����ȫ��Ӧʱ����ȥ�Ͻ�25g����ȥϡ����100g����������Ϊ25g��100g=1��4��
��Ͻ���п����������Ϊx��ϡ�������������������Ϊy��
Zn+H2SO4=ZnSO4+H2��
65 98 2
25g?x 100g?y 0.4g
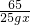 =
= =
=
x=52%��y=19.6%
�ʴ�Ϊ����1��52%����2��1��4��19.6%��
�������ɱ���һ���������ݶԱȿ�֪����������0.4gʱ��25g�Ͻ��Ѿ���ȫ��Ӧ��
�ɱ���һ���������ݶԱȿ�֪����������0.4gʱ��100gϡ�����Ѿ���ȫ��Ӧ����˵ڶ����еĺϽ��ϡ����ǡ����ȫ��Ӧ��
��������������������п�����ᷴӦ�Ļ�ѧ����ʽ���Լ�����Ͻ���п������������ϡ���������������������
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�
��Ͻ���п����������Ϊx��ϡ�������������������Ϊy��
Zn+H2SO4=ZnSO4+H2��
65 98 2
25g?x 100g?y 0.4g
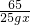 =
= =
=
x=52%��y=19.6%
�ʴ�Ϊ����1��52%����2��1��4��19.6%��
�������ɱ���һ���������ݶԱȿ�֪����������0.4gʱ��25g�Ͻ��Ѿ���ȫ��Ӧ��
�ɱ���һ���������ݶԱȿ�֪����������0.4gʱ��100gϡ�����Ѿ���ȫ��Ӧ����˵ڶ����еĺϽ��ϡ����ǡ����ȫ��Ӧ��
��������������������п�����ᷴӦ�Ļ�ѧ����ʽ���Լ�����Ͻ���п������������ϡ���������������������
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
Ϊ�ⶨijͭп�Ͻ���п������������ijͬѧ���úϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
��1���Լ���ͭп�Ͻ���п���������� ��
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ����������� ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ ��
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��������������/g | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������
��2002?�Ƹԣ�Ϊ�ⶨijͭп�Ͻ���п������������ijͬѧ���úϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
��1���Լ���ͭп�Ͻ���п����������______��
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��������������/g | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
 ��һ�о���ѧϰС��Ϊ�ⶨijͭп�Ͻ�ijɷ֣�ȡ10g�úϽ��������ձ��У��ټ���93.7g��������Ϊ20%��ϡ���ᣨ��Ӧ��������ʣ�ࣩ����Ӧ�������ձ��ڹ������ʵ������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺
��һ�о���ѧϰС��Ϊ�ⶨijͭп�Ͻ�ijɷ֣�ȡ10g�úϽ��������ձ��У��ټ���93.7g��������Ϊ20%��ϡ���ᣨ��Ӧ��������ʣ�ࣩ����Ӧ�������ձ��ڹ������ʵ������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺