��Ŀ����
ijС��ͬѧ������ͭ��ϡ���ᷴӦ����Ȥʵ�飬�����е�ͬѧ�õ���ɫ��Һ���е�ͬѧ�õ���ɫ��Һ����ʦ��ʾ������������������Ȼ�ͭ��Һ�йء����ǣ�ͬѧ�Ƕ�����������̽����
ʵ��1��̽��Ӱ���Ȼ�ͭ��Һ��ɫ������
ȡ��������������ͬ���Ȼ�ͭ��Һ��20 mL����ͬ�����ձ��У��ֱ����ڲ�ͬ�¶ȵ�ˮԡ���м���(�����ܼ�����)������Һ�¶Ⱥ㶨�۲���Һ��ɫ����¼���£�
�ձ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
������������/ % | 5 | 5 | 5 | 10 | 10 | 10 | 20 | 20 | 20 |
ˮԡ�� �¶�/��C | 30 | 50 | 70 | 30 | 50 | 70 | 30 | 50 | 70 |
��Һ��ɫ | dz�� | dz�� | �� | �� | ���� | ���� | ���� | ���� | ī�� |
ʵ��2��̽���������Ȼ�ͭ��Һ�ı�ɫŨ�ȷ�Χ
a �����£���ȡ15.4g���Ȼ�ͭ��������35.0gˮ�У����Ƴ�������������30.6%���Ȼ�ͭ��Һ��
b �������Ȼ�ͭ��Һ���μ����������ˮϡ�ͣ����Ƴɲ�ͬ���������������Ȼ�ͭ��Һ���۲���Һ��ɫ����¼���£�
������������/ % | 30.6 | 25.5 | 21.9 | 19.2 | 17.0 | 15.3 |
��Һ��ɫ | ���� | ���� | ���� | ���� | �� | dz�� |
����������ۣ�
(1)����ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ______��
(2)ʵ��1�У��ܢݢ�ʵ��Ŀ����______��
(3)����ʵ��1�Ľ����֪���ۢޢ���Ӱ���Ȼ�ͭ��Һ��ɫ��������______��
(4)ʵ��2�У�����a���õ��IJ�����������Ͳ���ιܡ���������________��
����˼�����ۣ�
(5)�����£�С�Ľ�20g�Ȼ�ͭ��������80gˮ�У������Һ����ɫΪ______��
(6)С�������һ����������Ϊ12%���Ȼ�ͭ��Һ����ҺΪdz��ɫ����Ҫʹ����Һ��������ɫת�䣬�ɽ��еIJ�����______(����ĸ���)��
A ����Һ����ˮԡ���м��ȵ�70��C
B �����������Ȼ�ͭ���岢ʹ֮�ܽ�
C ����������ˮϡ��
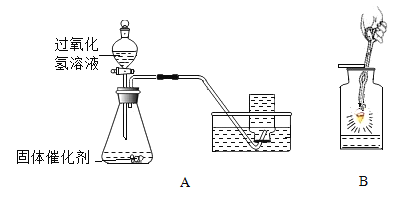
���A��B��������ѡ1����������������𣬰�A�Ʒ֡�ͼ��װ�õļг���������ȥ��
A����ͼװ���о����� | B����ͼװ���о�������̼ |
|
|
��1���������������Һ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ____�� ��2��ľ̿��������ȼ��ʱ��������____�� | ��1������ϡ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_____�� ��2��ʯ����ֽ����������_____�� |
��֪��20��ʱ��KCl��KNO3���ܽ�ȷֱ�Ϊ34 g��31.6 g������20��ʱ������������Һ��
| ��Һ | �� | �� | �� | �� |
�������� | KCl | KCl | KNO3 | KNO3 | |
������������/g | 20 | 35 | 20 | 35 | |
ˮ������/g | 100 | 100 | 100 | 100 |
�����й���Һ�١��ܵ�˵������ȷ����
A.���ڱ�����Һ���Ǣڢ�
B.��Һ��������=��
C.��Һ�����ʵ�����������=��
D.�����������ܼ���������Ϊ17:50


 B.
B.
 D.
D.