��Ŀ����
 ���⡢���������غ㶨����ѧ�û�ѧ�Ļ�����
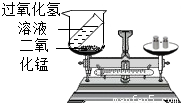
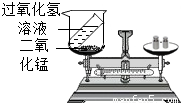
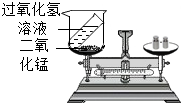
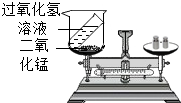
���⡢���������غ㶨����ѧ�û�ѧ�Ļ�������1��ijͬѧ����ͼװ�ö������غ㶨�ɽ���ʵ��̽�����۲쵽��Ӧ����ƽ��ƽ�⣬�����������غ㶨�ɽ�����ԭ����
��2���������غ㶨�ɿ�֪���ڻ�ѧ��Ӧǰ��һ���������
�ٷ������� ��ԭ������ �۷�����Ŀ
��ԭ����Ŀ ���������� ��Ԫ������
��3��4.14�������������������ɾ���ʧ��Ϊ��ֹ�ֺ������飬����������������������е�һ����������Ư�ۣ�������Ч�ɷ��Ǵ������[Ca��ClO��2]�������������ˮ�Ͷ�����̼������Ӧ������������Ӧ�Ļ�ѧ����ʽ��Ca��ClO��2+CO2+H2O=CaCO3��+2
��4��A��B��C�������ʸ�15g�����ǻ���ʱֻ����30g������D��������10gA����Ӧֹͣ��ԭ��Ӧ����ֻʣ��B���������������ƶ�����˵������ȷ����
A���÷�Ӧ�У�B�Ǵ��� B����һ�η�Ӧֹͣ��Cʣ��9g
C���ڶ��η�Ӧ��D������Ϊ50g D����Ӧ��A��B����������5��2
E����Ӧ��A��C����������5��2��
��������1���ڻ�ѧ��Ӧ�У��μӷ�Ӧǰ�����ʵ������ܺ͵��ڷ�Ӧ�����ɸ����ʵ������ܺͣ���ͽ��������غ㶨�ɣ���������ֽⷴӦ������H2O��O2��
��2�����������غ㶨�ɣ���ѧ��Ӧ�Ĺ��̣����Dzμӷ�Ӧ�ĸ����ʣ���Ӧ���ԭ�ӣ�������϶������������ʵĹ��̣��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣻
��3�����������غ㶨�ɿ�֪���ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮���ݴ��������ˮ�Ͷ�����̼������Ӧ�Ļ�ѧ����ʽ�������غ㶨�ɣ��Ϳɼ�����������Ԫ�ص�ԭ�Ӹ����뷴Ӧ���Ԫ�ص�ԭ�Ӹ���֮�Ȼ�����2��������ȱ�Ļ�ѧʽ�к��е�Ԫ�ص�ԭ�Ӹ�����
��4�����������뷴Ӧ�����������غ㶨�ɺ����ʵ�������ϵ����ѡ����һ������������ȷѡ��
��2�����������غ㶨�ɣ���ѧ��Ӧ�Ĺ��̣����Dzμӷ�Ӧ�ĸ����ʣ���Ӧ���ԭ�ӣ�������϶������������ʵĹ��̣��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣻
��3�����������غ㶨�ɿ�֪���ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮���ݴ��������ˮ�Ͷ�����̼������Ӧ�Ļ�ѧ����ʽ�������غ㶨�ɣ��Ϳɼ�����������Ԫ�ص�ԭ�Ӹ����뷴Ӧ���Ԫ�ص�ԭ�Ӹ���֮�Ȼ�����2��������ȱ�Ļ�ѧʽ�к��е�Ԫ�ص�ԭ�Ӹ�����
��4�����������뷴Ӧ�����������غ㶨�ɺ����ʵ�������ϵ����ѡ����һ������������ȷѡ��
����⣺��1�����������غ㶨�ɣ��÷�Ӧ�вμӷ�Ӧ��H2O2���������ڷ�Ӧ�����ɵ�H2O��O2������֮�ͣ���ʵ��δ���ܱ������н��У�O2��ɢ�������У�������ƽ��ƽ�⣻������Ӧ�Ļ�ѧ����ʽ��2H2O2
2H2O+O2����
��2�����������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࡢԭ����Ŀ��Ԫ�����ࣻ��ѡ���ڢܢޣ�
��3�����������غ㶨�ɺͻ�ѧ����ʽ��֪����ȱ�Ļ�ѧʽ�к���Ca�ĸ���Ϊ��1-1=0������Cl�ĸ���Ϊ��2��2=1������C�ĸ���Ϊ��1-1=0������O�ĸ���Ϊ����2+2+1-3����2=1������H�ĸ���Ϊ��2��2=1���ʸû�ѧ����ʽ����ȱ�Ļ�ѧʽΪ��HClO��
��4��A�����ݡ�������10gA����Ӧֹͣ��ԭ��Ӧ����ֻʣ��B����֪��A��C��������Ϊ��15g+10g����15g=5��3�����һ�η�Ӧֹͣ��C���뷴Ӧ������Ϊ��15g��5��3=9g����ôB���뷴Ӧ������Ϊ��30g-15g-9g=6g����B���Ǵ�������A���ʺϣ�
B�����ݡ�������10gA����Ӧֹͣ��ԭ��Ӧ����ֻʣ��B����֪��A��C��������Ϊ��15g+10g����15g=5��3�����һ�η�Ӧֹͣ��Cʣ�������Ϊ��15g-��15g��3��5��=6g����B���ʺϣ�
C��������֪��������һ��A��ȫ��Ӧ������D������Ϊ30g����A��D��������Ϊ��15g��30g=1��2���ڶ��η�Ӧ��D������Ϊ����10g+15g����2=50g����C�ʺϣ�
D������A���������ݿ�֪����Ӧ��A��B���������ǣ�15g��6g=5��2����D�ʺϣ�
E������B���������ݿ�֪����Ӧ��A��C���������ǣ�15g��9g=5��3����E���ʺϣ�
��ѡCD��
�ʴ�Ϊ����1�����������غ㶨�ɣ��÷�Ӧ�вμӷ�Ӧ��H2O2���������ڷ�Ӧ�����ɵ�H2O��O2������֮�ͣ���ʵ��δ���ܱ������н��У�O2��ɢ�������У�������ƽ��ƽ�⣨�÷�Ӧ���������ɻ�ʵ��δ���ܱ������н��У��𰸺������֣���2H2O2
2H2O+O2����
��2���ڢܢޣ�
��3��HClO��
��4��CD��
| ||
��2�����������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��һ��������ǣ�ԭ�����ࡢԭ����Ŀ��Ԫ�����ࣻ��ѡ���ڢܢޣ�
��3�����������غ㶨�ɺͻ�ѧ����ʽ��֪����ȱ�Ļ�ѧʽ�к���Ca�ĸ���Ϊ��1-1=0������Cl�ĸ���Ϊ��2��2=1������C�ĸ���Ϊ��1-1=0������O�ĸ���Ϊ����2+2+1-3����2=1������H�ĸ���Ϊ��2��2=1���ʸû�ѧ����ʽ����ȱ�Ļ�ѧʽΪ��HClO��
��4��A�����ݡ�������10gA����Ӧֹͣ��ԭ��Ӧ����ֻʣ��B����֪��A��C��������Ϊ��15g+10g����15g=5��3�����һ�η�Ӧֹͣ��C���뷴Ӧ������Ϊ��15g��5��3=9g����ôB���뷴Ӧ������Ϊ��30g-15g-9g=6g����B���Ǵ�������A���ʺϣ�
B�����ݡ�������10gA����Ӧֹͣ��ԭ��Ӧ����ֻʣ��B����֪��A��C��������Ϊ��15g+10g����15g=5��3�����һ�η�Ӧֹͣ��Cʣ�������Ϊ��15g-��15g��3��5��=6g����B���ʺϣ�
C��������֪��������һ��A��ȫ��Ӧ������D������Ϊ30g����A��D��������Ϊ��15g��30g=1��2���ڶ��η�Ӧ��D������Ϊ����10g+15g����2=50g����C�ʺϣ�
D������A���������ݿ�֪����Ӧ��A��B���������ǣ�15g��6g=5��2����D�ʺϣ�
E������B���������ݿ�֪����Ӧ��A��C���������ǣ�15g��9g=5��3����E���ʺϣ�
��ѡCD��
�ʴ�Ϊ����1�����������غ㶨�ɣ��÷�Ӧ�вμӷ�Ӧ��H2O2���������ڷ�Ӧ�����ɵ�H2O��O2������֮�ͣ���ʵ��δ���ܱ������н��У�O2��ɢ�������У�������ƽ��ƽ�⣨�÷�Ӧ���������ɻ�ʵ��δ���ܱ������н��У��𰸺������֣���2H2O2
| ||
��2���ڢܢޣ�
��3��HClO��
��4��CD��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
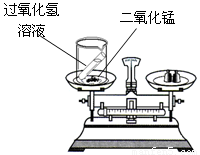
 ���⡢���������غ㶨����ѧ�û�ѧ�Ļ�����
���⡢���������غ㶨����ѧ�û�ѧ�Ļ�����