��Ŀ����

(2)����������������ɫ����ʱ�������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ�������_________��
(3)�����ܵ��Թ��в���������ʹ�����ǵ�ľ����ȼ���������Ϊ__________��
(4)����д����pH��ֽ�����Һ����Եľ��������____________��
(2)̿(��̼����C)
(3)������O2
(4)��ȡС��pH��ֽ���ڽྻ������(���ΰ塢����Ƭ)�ϣ��ò�����պȡ(���ý�ͷ�ι�ȡ)������Һ(����Һ������Һ)��(���)��pH��ֽ�ϣ���ֽ��ɫ�������ɫ���Ƚ϶�����Ӧ��ֵ��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
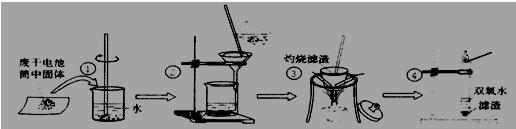
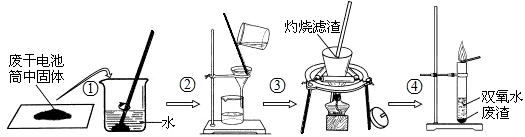
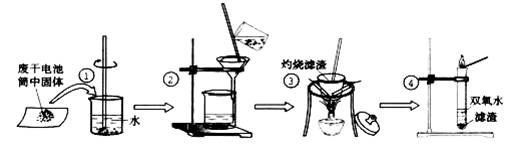
��ĩ�����ϵ�д�ijС��ͬѧ�Էϸɵ��Ͳ�ڵĺ�ɫ���壨�ں���MnO2��NH4Cl��ZnCl2�ȣ�����������ͼ��ʾ��ʵ�飺
��1������������������ɫ����ʱ�������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ�������________��
��2����֪���Ȼ�п��Һ����ε���ϡ��ˮ��������Zn��OH��2��ɫ������Ȼ���ܽ����ɿ�����ˮ��Zn��NH3��4Cl2��ͬѧ�ǶԲ����ڵ���Һ�еijɷֽ���̽����
| ��֤���� | ���� | �ж� | |
| ����I����Ҫ�ɷ�Ϊ�Ȼ�� | ȡ������Һ�����������ƹ��壬������ | δ�ŵ������Ĵ̼�����ζ | ________ |
| ����II����Ҫ�ɷ��Ȼ�п | ________ �������������� | ��ʼ���ְ�ɫ�����������������ܽ� | ����II���� |
| ������ʵ��ó��Ľ����ǣ���Һ��________�� | |||
ijС��ͬѧ�Էϸɵ��Ͳ�ڵĺ�ɫ����(�ں���MnO2��NH4Cl��ZnCl2��)����������ͼ��ʾ��ʵ�飺
(1)�����ڵ������� ���ò����в������������� ��
(2)����������������ɫ����ʱ�������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ������� ��
(3)�����ܵ��Թ��в���������ʹ�����ǵ�ľ����ȼ���������Ϊ ��
(4)����д����pH��ֽ�����Һ����Եľ��������
��
(5)��֪���Ȼ�п��Һ����ε���ϡ��ˮ��������Zn(OH)2��ɫ������Ȼ���ܽ����ɿ�����ˮ��Zn(NH3)4Cl2��ͬѧ�ǶԲ����ڵ���Һ�еijɷֽ���̽����
| ��֤���� | ���� | �ж� | |
| ����I����Ҫ�ɷ� Ϊ�Ȼ�� | ȡ������Һ����NaOH���壬������ | δ�ŵ����Եİ��� �̼�����ζ |
|
| �������Ҫ�ɷ� Ϊ�Ȼ�п |
|
| �������� |
| ������ʵ��ó��Ľ����ǣ���Һ�� | |||