��Ŀ����
������в�����գ�����һ��2009��1�£�ij�оưɷ���һ���ش���֣����¹ʵ������Ǽ����������ھư���ȼ���̻�����ȼ�۰�����ĭ�����컨�壬�۰�����ĭ����ȼ�ղ��������ж������������Ա�ж�������
���϶���������þ��һ�����͵���ȼ�������¶ȴﵽ380��ʱ��������þ��ʼ�ֽ��ˮ������ͬʱ�������µ�����þ���壮����������þ��һ���ʣ��������������ϵ���ȼ�Բ�����������ȼ���ã�
��1���۰�����ĭ���� �����л��߷��Ӳ��ϣ���ǡ�����
��ش�۰�����ĭ���ϵ�һ�㻯ѧ���� ��
��2��ȼ�ŵ��̻��������ⳡ����������������� �������ţ�
A���ṩ����B��ʹ��ȼ����¶ȴﵽ�Ż��C���ṩ��ȼ��
��3���������ԭ����������������þ������ȼ����ԭ�� ������һ�㣩
������þ���ȷֽ�Ļ�ѧ����ʽΪ ��
���𰸡���������1���۰��������л��߷��Ӻϳɲ��ϣ�������Ϣ�ж����ֲ����п�ȼ�ԣ�
��2���̻����˵�ȼ�����ã�Ҳ�����ṩ������ȼ��ʱ���¶ȣ�
��3����������ԭ���жϣ������߿�ȼ���ڸ���������۽������Ż�����£����ݷ�Ӧ��������д����Ӧ��ѧ����ʽ��
����⣺��1���۰����Ǿۺ�������л��߷��Ӻϳɲ��ϣ����ֲ����п�ȼ�ԣ�
��2���̻����˵�ȼ�۰��������ã�Ҳ�����ṩ����ȼ��ʱ���¶ȣ�ʹ�۰�����ĭ���ϵ��¶ȴﵽ�Ż���������֣�
��3��������þ�ֽ���������þ��ˮ����ѧ����ʽΪ��������ȼ�յ���������������֪380��������������þ�����ֽ�����ȼ���������������ͷų�����ˮ�֣��ֽ��������þ�����ڿ�ȼ����棬��ֹȼ�յĽ��У�������þ���ȷֽ�Ļ�ѧ����ʽΪ��Mg��OH��2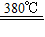 MgO+H2O����
MgO+H2O����
�ʴ�Ϊ����1���� ��ȼ�� ��2��B
��3��������þ���ȷֽ����ɵ�����þ��ˮ������������ȼ������棬ʹȼ����������������
Mg��OH��2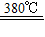 MgO+H2O����
MgO+H2O����
�����������ǽ��ȼ�յ����������Ĵ�ʩ�������л�ѧ�Ŀ��飬��������ṩ����Ϣ�����صĻ�ѧ֪ʶ�ǽ���Ĺؼ���
��2���̻����˵�ȼ�����ã�Ҳ�����ṩ������ȼ��ʱ���¶ȣ�
��3����������ԭ���жϣ������߿�ȼ���ڸ���������۽������Ż�����£����ݷ�Ӧ��������д����Ӧ��ѧ����ʽ��
����⣺��1���۰����Ǿۺ�������л��߷��Ӻϳɲ��ϣ����ֲ����п�ȼ�ԣ�
��2���̻����˵�ȼ�۰��������ã�Ҳ�����ṩ����ȼ��ʱ���¶ȣ�ʹ�۰�����ĭ���ϵ��¶ȴﵽ�Ż���������֣�
��3��������þ�ֽ���������þ��ˮ����ѧ����ʽΪ��������ȼ�յ���������������֪380��������������þ�����ֽ�����ȼ���������������ͷų�����ˮ�֣��ֽ��������þ�����ڿ�ȼ����棬��ֹȼ�յĽ��У�������þ���ȷֽ�Ļ�ѧ����ʽΪ��Mg��OH��2
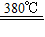 MgO+H2O����
MgO+H2O�����ʴ�Ϊ����1���� ��ȼ�� ��2��B
��3��������þ���ȷֽ����ɵ�����þ��ˮ������������ȼ������棬ʹȼ����������������
Mg��OH��2
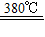 MgO+H2O����
MgO+H2O���������������ǽ��ȼ�յ����������Ĵ�ʩ�������л�ѧ�Ŀ��飬��������ṩ����Ϣ�����صĻ�ѧ֪ʶ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ