��Ŀ����
����Ŀ�����������������е�Ӧ��Խ��Խ�㷺����ش�����������⣺
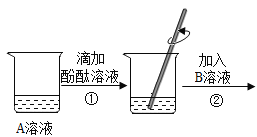
��1��ͭ�������������ߣ���Ϊ��������õ�__________��
��2�������������Ͻ���̻����������кۼ������Ͻ���û�У�˵��������Ӳ��__________����������������С���������Ͻ�
��3������������������⣬�������ʵ������������е�__________��__________�ȷ�����ѧ��Ӧ��
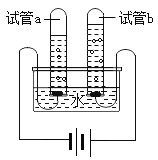
��4��ʵ����֤п��ͭ�������ֽ������ǿ���ķ����ж��֣����磺�����ֽ����ֱ�Ͷ�뵽__________���ѧʽ����Һ�м�����֤��
��5����ͼ����һ������������Һ�м���һ������ͭ��п�Ļ�����ĩ����ַ�Ӧ����ˣ��õ���Һ�����ҡ�
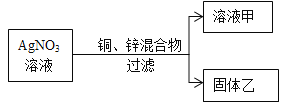
������Һ��Ϊ��ɫ�����������һ�����е�������__________���ѧʽ��
�����������к���п��ͭ�������֣�����Һ���������Ľ������ӷ���Ϊ__________����ʱ��������м���ϡ���ᣬ������Ӧ�Ļ�ѧ����ʽΪ__________��
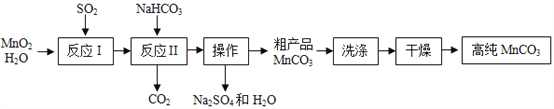
��6����ͨ��ͭ����ͭ��п��ɵĺϽ𡣳�ȡ200g��ͨ��ͭ�ķ�ĩ��Ʒ������������ϡ�����ַ�Ӧ���˳�ʣ��Ĺ��壬������ϴ�ӡ��������������Ϊ13.5g��ͨ������ó����úϽ���п������������__________�����ĵ���������Ϊ__________g��
���𰸡������� С�� ���� ˮ���� Cu(NO3)2�������𰸾��� �� Ag Zn2+ Zn+H2SO4=ZnSO4+H2�� 93.25�� 281.2
��������
��1��ͭ�������������ߣ���Ϊ��������õĵ����ԣ���������ԡ�
��2�������������Ͻ���̻����������кۼ������Ͻ���û�У�˵��������Ӳ��С�����Ͻ𣬹��С�ڡ�
��3������������������⣬�������ʵ������������е�ˮ�����������ȷ�����ѧ��Ӧ�����ˮ������������
��4����п��ͭ�������ֽ����ֱ�Ͷ�뵽Cu(NO3)2��Һ�У�Zn��Cu(NO3)2��Ӧ����Cu��Zn(NO3)2��˵���������Zn��Cu��Ag��Cu(NO3)2����Ӧ��˵���������Ag��Cu���ʽ������Zn��Cu��Ag,���Cu(NO3)2
��5������������Һ�м���һ������ͭ��п�Ļ�����ĩ����Ϊ�������Zn��Cu��Ag���������Ⱥ�п��Ӧ�ٺ�ͭ��Ӧ��п����������Ӧ��������п��������Һ��һ��������п������һ������������Һ��Ϊ��ɫ˵����Һ��������ͭ����������һ��û��п������Һһ��������п������ͭ������һ������һ��ûп�����Ag��
�����������к���п��ͭ�������֣�����Һһ��û������ͭ����������һ��������п����Һ��һ���н�������ΪZn2+����ʱ��������м���ϡ���ᣬп��ϡ���ᷴӦ��������п��������������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4=ZnSO4+H2����
��6����Ӧ�����Ϊͭ����п������Ϊ200g-13.5g=186.5g����������Ϊ��![]() ����������������Ϊx��
����������������Ϊx��

���93.25%��281.2

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
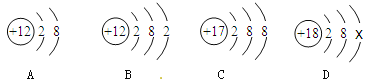
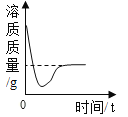
�ۺ��Բ�ϵ�д�����Ŀ������ͼ������ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
A��һ�����Ķ��������м������������Һ | B����һ�����ĸ�����ع��� | C��һ���� AgNO3�� Cu(NO3)2 �Ļ����Һ�м������� | D�� NaOH ��Һ�еμ�ϡ���������� |
A.AB.BC.CD.D