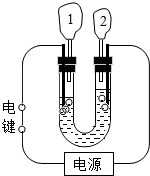
��Ŀ����
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϣ�һͬѧ��ij�ִ��ν����ᴿʵ�飬������ͼ��ʾ��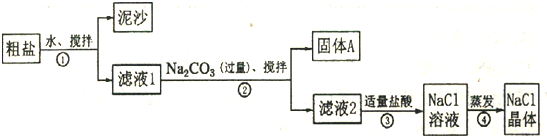
��ش�
��1������ٺ͢ڵIJ���������______��
��2������ܼ�������ʱҪ�õ����������Ͻ��裬����Ϊ�˷�ֹ______��
��3���������֤��
��4����ʡ������ij�ָ��ε����ϱ����£��Ȼ��ơ�ʳ��̼��ơ�����أ�Ϊ�˲ⶨ�����еĸ�Ԫ�غ�����ȡ10g����������ˮ�������������ᣬ����0.132g������̼�������˼Ӹ�ʳ���и�Ԫ�ص�����������

��ش�
��1������ٺ͢ڵIJ���������______��
��2������ܼ�������ʱҪ�õ����������Ͻ��裬����Ϊ�˷�ֹ______��
��3���������֤��
| ���� | ��֤���� | ʵ������ | ���� |
| �������A�к� CaCO3 | ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿ� | ______ | �������� |
| �������A�к� BaCO3 | ȡ��������A���Թ��У��ȵ���______���ٵ���Na2SO4��Һ | �����ݷų����ް�ɫ���� | ______ |
| ���������Ƶõ� NaCl�����л�����Na2SO4 | ȡ����NaCl�������Թ��У�����������ˮ�ܽ⣬��______ | ______ | �������� |
��1���Ѳ�����Һ��Ĺ����Һ�����ķ������ǹ��ˣ��ʴ�Ϊ�����ˣ�
��2���ڸò����У��ò��������裬�Ƿ�ֹ��Һ�ֲ����ȶ��ɽ����ʴ�Ϊ����Һ�ֲ����ȶ��ɽ���
��3�������̼������ӵļ��鷽�����ǵμ�ϡ���ᣬ��������ܹ�ʹ����ʯ��ˮ����ǵ����壬˵����̼������ӣ����������������ݲ�����ʯ��ˮ����ǣ��ʴ�Ϊ�������ݲ�����ʯ��ˮ����ǣ�
������������֪û�б����ӣ�Ӧ���ȼ������ϡ���ᣬ�ṩ�����������ټ������û�г���˵��û�б����ӣ����ڶ��ֲ�����ʴ�Ϊ��ϡ����������
������������Ӧ�ü������ᱵ��ϡ���ᣬ����г�������˵���������ƣ��ʴ�Ϊ����BaCl2���а�ɫ�������ɣ�
��4����˼Ӹ�ʳ����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
10044
x0.132g
100��44=x��0.132g
x=0.3g
̼����иƵ�����Ϊ0.3g��
��100%=0.12g
�˼Ӹ�ʳ���и�Ԫ�ص���������Ϊ��
��100%=1.2%
�𣺸�ʳ���и�Ԫ�ص���������1.2%��
��2���ڸò����У��ò��������裬�Ƿ�ֹ��Һ�ֲ����ȶ��ɽ����ʴ�Ϊ����Һ�ֲ����ȶ��ɽ���
��3�������̼������ӵļ��鷽�����ǵμ�ϡ���ᣬ��������ܹ�ʹ����ʯ��ˮ����ǵ����壬˵����̼������ӣ����������������ݲ�����ʯ��ˮ����ǣ��ʴ�Ϊ�������ݲ�����ʯ��ˮ����ǣ�
������������֪û�б����ӣ�Ӧ���ȼ������ϡ���ᣬ�ṩ�����������ټ������û�г���˵��û�б����ӣ����ڶ��ֲ�����ʴ�Ϊ��ϡ����������
������������Ӧ�ü������ᱵ��ϡ���ᣬ����г�������˵���������ƣ��ʴ�Ϊ����BaCl2���а�ɫ�������ɣ�
��4����˼Ӹ�ʳ����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
10044
x0.132g
100��44=x��0.132g
x=0.3g
̼����иƵ�����Ϊ0.3g��
| 40 |
| 100 |
�˼Ӹ�ʳ���и�Ԫ�ص���������Ϊ��
| 0.12g |
| 10 |
�𣺸�ʳ���и�Ԫ�ص���������1.2%��

��ϰ��ϵ�д�
�����Ŀ