��Ŀ����
����Ŀ�����µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��ء���������ѧ��ѧ֪ʶ�ش����⡣
��1��Ŀǰú̿���ҹ���Դ�ṹ��ռ�еı������
���ҹ������ƹ��ͥ������Ȼ������ú��ȼ�ϣ���Ȼ������Ҫ�ɷ���_______________��
�����겻����ֲ������������úȼ�ղ�����_____________�����������γɡ������͡����ꡣ
��2�����ೱ������ˮ������Ҫ��ָˮ��ijЩֲ��Ӫ��Ԫ�غ������ߣ�����ˮ���ϵ���������������ֳ��ˮ�ʶ��������������
����֪ij���ຬ�л�ѧʽΪC106H263O106N16P����Է�������Ϊ3486�����ʣ��������е�Ԫ�ص���������Ϊ______________����ȷ��0.1%�����������������ʵĻ�ѧʽ����ȷ����ҵ��������ˮ�е����������������Ӫ��Ԫ����_______________����Ԫ�����ƣ���
�ڽ��ˮ����Ⱦ��Ӧ�ӿ�����ȾԴͷ���֡����д�ʩ�ܷ�ֹ���ೱ������ˮ��������������__________������ţ���
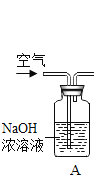
A ��ˮ���ذ��½���סլС�������ݡ����ꡢҽԺ��Ҫ����������ˮ����װ�ã��Ӹ����Ͻ��������ˮ����ˮ�������
B �ذ����о����ֹʹ�ú���ϴ�·�
C ��ֹ�ø�ˮ���ˮ���ũ�������Ϊ������ˮ
D ���ˮ���м�������ľ�ˮ�����������Ը���ˮ�ʡ�
��3���������ɴ��ס��ҵ��ס����嵰���������ֵ�����Ƴɵķۼ�������;��ΪӪ�����������µĵ�����ȱ������Ⱥ����Ӫ������ش�
�ٵ��۲����Ӫ���ɷ���___________________��
���������ʵ�������������ʳ���㾫�����ۣ�����ɫ��ζ���϶����Էֱ档��������Ʒ��֤���ֵ����к��е��۵ķ�����__________________��
���𰸡�CH4 SO2 6.4% ��Ԫ�غ���Ԫ�� AB ������ �μӵ�Һ
��������
��1������Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽΪCH4��
�����겻����ֲ������������úȼ�ղ����Ķ�������ѧʽΪSO2�������������γɡ������͡����ꡣ
��2������֪ij���ຬ�л�ѧʽΪC106H263O106N16P����Է�������Ϊ3486�����ʣ��������е�Ԫ�ص���������Ϊ![]() �����ຬ�л�ѧʽΪC106H263O106N16P�����е�Ԫ�غ���Ԫ�أ����������������ʵĻ�ѧʽ����ȷ����ҵ��������ˮ�е����������������Ӫ��Ԫ���ǵ�Ԫ�غ���Ԫ�ء�
�����ຬ�л�ѧʽΪC106H263O106N16P�����е�Ԫ�غ���Ԫ�أ����������������ʵĻ�ѧʽ����ȷ����ҵ��������ˮ�е����������������Ӫ��Ԫ���ǵ�Ԫ�غ���Ԫ�ء�
��A����ˮ���ذ��½���סլС�������ݡ����ꡢҽԺ��Ҫ����������ˮ����װ�ã��Ӹ����Ͻ��������ˮ����ˮ������⣬�ܷ�ֹ���ೱ������ˮ��������������A��ȷ��
B���ذ����о����ֹʹ�ú���ϴ�·ۣ��ܷ�ֹ���ೱ������ˮ��������������B��ȷ��
C����ֹ�ø�ˮ���ˮ���ũ�������Ϊ������ˮ�����ڴӿ�����ȾԴͷ���֣���C����ȷ��
D�����ˮ���м�������ľ�ˮ�����������Ը���ˮ�ʣ�����������ʩ�������ڴӿ�����ȾԴͷ���֣���D����ȷ����ѡAB��
��3��������������Ӫ�����ǵ����ʡ�ˮ�����Ρ�ˮ��֬����ά���أ��ʵ��۲����Ӫ���ɷ��ǵ����ʡ�
�ڵ������۱���ɫ������������Ʒ��֤���ֵ����к��е��۵ķ����ǵμӵ�Һ��

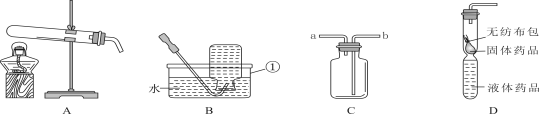
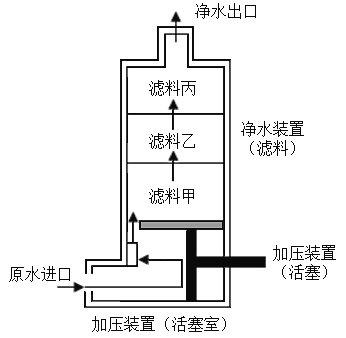
����Ŀ��Ϊ�˽��������Ұ���������ʱ����ˮ���⣬С��չ�˱�ЯʽҰ�⾻ˮ������ƺ���������Ʒ�����ͼ��ʾ�����м�ͷ��ʾԤ���ˮ������Ϊ��ʹˮ�ʷ�������ˮ����С��ѡ������������ȥ��ԭˮ�е����ʣ��������ϵ���;�����ʾ�������ϼס��ҡ����ֱ�Ϊ_____��������д��ţ���
���ϱ�� | ��; |
�� | ȥ��ˮ�е���ɳ��������ȴ������Ⱦ�� |
�� | ȥ��������л���ؽ������Ӻ�ϸ���� |
�� | ȥ��������ϸ����С�����л���ؽ������Ӻ���ζ�� |
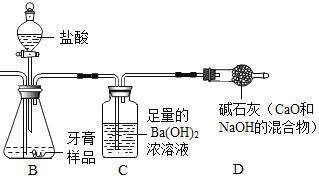
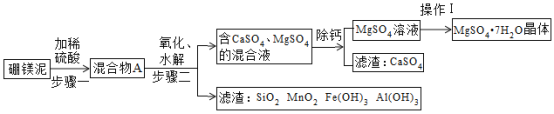
����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%��������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʡ�����þ��Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO4��7H2O�ļ�Ҫ��ҵ��������:
��������ش���������:
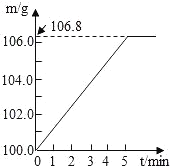
��1��ʵ������Ҫ��98%��Ũ���ᣨp=1.84g/mL��������һ��Ũ�ȵ�ϡ���ᡣ���������������У��������ƹ����б���ʹ�õ���_____________����дѡ����ĸ����
A ��ͨ©��
B ������
C ��Ͳ
D �ձ�
E ������
F ҩ��
��2�������A�У�����Mg2+��Ca2+��Mn2+��Fe2+�������еĽ�����������______________�������ӷ��ţ���
��3������������Ĺ��̣����ϼ۷����仯�����ֽ���Ԫ����______________����Ԫ�ط��ţ���
��4����֪MgSO4��CaSO4�ܽ�����±���
�¶ȣ��棩 | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�ٴӱ������ݿ����ܽ��CaSO4�ܽ�����¶ȵı仯������___________________��
�����������ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݷ����������������˵��¶���______________(�����ϵ��¶��������ϸ��¶�������
��5��������I���ǽ���Һ��������Ũ������ȴ�ᾧ��________________����õ�MgSO4��7H2O��
����Ŀ����ʵ���ң�С����ϡ���ᵹ��Ba(OH)2��Һ�еõ���Һ�ף����ʵ��̽�����¡�
��������⣩��ַ�Ӧ�����ü���Һ�е�������ʲô��
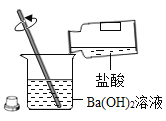
���������룩��1���Ȼ���
��2���Ȼ���������
��3��_____________________��
��ʵ�鷽������4��̽������Һ���Ƿ������
ʵ�� | ���� | ���� | ���� |
�� | ȡ��������Һ���Թ��У� ______________________�� | û�����ݲ��� | ���в������� |
��5��̽������Һ���Ƿ�������������
ʵ�� | ���� | �����뻯ѧ����ʽ | ���� |
�� | ȡ��������Һ���Թ��У������������Ȼ�����Һ�� | _________ | ���к��������� |
����һ���Լ�һ����ȷ������Һ���Ƿ����������������õ��Լ���___________________�� A���� B���� C�Ȼ�ͭ D������ | |||
���ó����ۣ���6��ͨ�������ʵ�飨4����5���Ʋ⣬�����������_______________________��