��Ŀ����
���µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ�Ļ�ѧ֪ʶ�ش��������⣺
��1�����п�չ�ˡ��崴���ʡ����Ϊ���������������������������ǵ�ǰ��Ҫ�Ĺ���֮һ�����г��ֵ�ijЩ��������Ҳ���������ǵĸ߶ȹ�ע��
�����Һ��ذ������кܴ���ƣ�����ˮ����Ⱦ��Ȼ���أ�Ϊ�о���Ⱦ�����Сǿͬѧ����pH��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
�ں���PM2.5�Զ��������ڽ���Ԫ�·�չ����ũ��ũ����ոѷ��ս��ܵ���һ����⡢Ԥ������Ԥ��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��
������β����ת�����з������з�Ӧ��2NO2+4CO
N2+4CO2���ڸ÷�Ӧ�л��ϼ۱仯��Ԫ��Ϊ
��2����ɫ���˵ġ���ɽˮ�⡱����������ˮ������ҪˮԴ������ˮ��������������ͼ��ʾ��
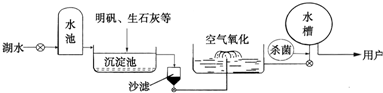
��������������ˮ����ʹ�õľ�ˮ������
A��ɱ�� B������ C����� D������
�ڼ�����ɽˮ��ˮ��Ӳˮ������ˮ�ļ���
��3�����д�ͳ���ˡ���ɽ���뼦Ƭ���뺼�ݡ�����Ϻ�ʡ�һ������ȫ�������ü���;�ɽ����軬�����ɣ�����ʳ�ü�����Բ����Ӫ������Ҫ��
��1�����п�չ�ˡ��崴���ʡ����Ϊ���������������������������ǵ�ǰ��Ҫ�Ĺ���֮һ�����г��ֵ�ijЩ��������Ҳ���������ǵĸ߶ȹ�ע��
�����Һ��ذ������кܴ���ƣ�����ˮ����Ⱦ��Ȼ���أ�Ϊ�о���Ⱦ�����Сǿͬѧ����pH��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
�ڰ״�Ƭ����Ƭ�Ϸ�һСƬpH��ֽ��������ҺͿ����ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ��ó�����Һ��pH��������������������
�ڰ״�Ƭ����Ƭ�Ϸ�һСƬpH��ֽ��������ҺͿ����ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ��ó�����Һ��pH��������������������
���ں���PM2.5�Զ��������ڽ���Ԫ�·�չ����ũ��ũ����ոѷ��ս��ܵ���һ����⡢Ԥ������Ԥ��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��
����
����
������ϡ������ֽ⡱�����û����������ֽ⡱����������֮һ����������β����ת�����з������з�Ӧ��2NO2+4CO
| ||
̼����
̼����
����дԪ�����ƣ�����2����ɫ���˵ġ���ɽˮ�⡱����������ˮ������ҪˮԴ������ˮ��������������ͼ��ʾ��

��������������ˮ����ʹ�õľ�ˮ������
ABC
ABC
��������ţ�A��ɱ�� B������ C����� D������
�ڼ�����ɽˮ��ˮ��Ӳˮ������ˮ�ļ���
ȡ�����������ˮ�������۲�����
ȡ�����������ˮ�������۲�����
����3�����д�ͳ���ˡ���ɽ���뼦Ƭ���뺼�ݡ�����Ϻ�ʡ�һ������ȫ�������ü���;�ɽ����軬�����ɣ�����ʳ�ü�����Բ����Ӫ������Ҫ��
������
������
����ɽ������к��ж����أ�C15H14O6?H2O������������3
3
��Ԫ����ɣ���������1������Һ�����Ȳⶨ����ʹ��pH��ֽ���ⶨ��Һ�����ȣ�����ⶨ�������ø���IJ�����պȡ����ͷ�ι���ȡ�������Ĵ�����Һ�������ڷ��ڸ���IJ������״ɰ��ϵĸ���pH��ֽ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������Һ��pH��pHֵ��7Ϊ���ԣ�pHֵ=7Ϊ���ԣ�pHֵ��7Ϊ���ԣ�
��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��������Ӧ��
�۸��ݸ����ʵĻ�ѧʽ���Լ���������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�жϳ�����Ԫ�صĻ��ϼۣ�
��2��������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
�ڸ���Ӳˮ����ˮ�����ַ������з�����
��3������������������Ӫ���ص����ࡢʳ����Դ���������������ʳ���ж�������Ӫ���أ����з����жϣ�
�ɶ����صķ���ʽC15H14O6?H2O�����ж������������е�Ԫ�ص�������
��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��������Ӧ��
�۸��ݸ����ʵĻ�ѧʽ���Լ���������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�жϳ�����Ԫ�صĻ��ϼۣ�
��2��������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
�ڸ���Ӳˮ����ˮ�����ַ������з�����
��3������������������Ӫ���ص����ࡢʳ����Դ���������������ʳ���ж�������Ӫ���أ����з����жϣ�
�ɶ����صķ���ʽC15H14O6?H2O�����ж������������е�Ԫ�ص�������
����⣺��1����Ҫ��pH��ֽ���Բⶨ��ˮ�������ǿ�������ڰ״�Ƭ����Ƭ�Ϸ�һСƬpH��ֽ��������ҺͿ����ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�����Һ��pH��
��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��������
�۸��ݵ�����Ԫ�صĻ��ϼ�Ϊ0���Լ���������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��CO��̼�Ļ��ϼ�Ϊ+2�����Ļ��ϼ�Ϊ-2��NO2�е��Ļ��ϼ�Ϊ+4�����Ļ��ϼ�Ϊ-2��CO2��̼�Ļ��ϼ�Ϊ+4�����Ļ��ϼ�Ϊ-2��N2�е��Ļ��ϼ�Ϊ0����Ԫ�ػ��ϼ۱仯����C��N��
��2��������ˮ����ʹ�õľ�ˮ�����г��������ˡ���������еȣ�
�ڼ���Ӳˮ����ˮ�ļ����ǣ�ȡ�����������ˮ���������϶��ˮ��Ӳˮ���������ˮ�����϶���ĭ��ˮ����ˮ��
��3��ʳ�ü����и����е����ʣ�
�����صķ���ʽ��C15H14O6?H2O������������C��H��O����Ԫ�أ�
�ʴ��ǣ���1�����ڰ״�Ƭ����Ƭ�Ϸ�һСƬpH��ֽ��������ҺͿ����ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ��ó�����Һ��pH���������������������� ����������̼����
��2��ABC��ȡ�����������ˮ�������۲�����
��3�������ʡ�3��
��ֲ��ոѷ��չ����з����ķ�Ӧ��Ҫ��������
�۸��ݵ�����Ԫ�صĻ��ϼ�Ϊ0���Լ���������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��CO��̼�Ļ��ϼ�Ϊ+2�����Ļ��ϼ�Ϊ-2��NO2�е��Ļ��ϼ�Ϊ+4�����Ļ��ϼ�Ϊ-2��CO2��̼�Ļ��ϼ�Ϊ+4�����Ļ��ϼ�Ϊ-2��N2�е��Ļ��ϼ�Ϊ0����Ԫ�ػ��ϼ۱仯����C��N��
��2��������ˮ����ʹ�õľ�ˮ�����г��������ˡ���������еȣ�
�ڼ���Ӳˮ����ˮ�ļ����ǣ�ȡ�����������ˮ���������϶��ˮ��Ӳˮ���������ˮ�����϶���ĭ��ˮ����ˮ��
��3��ʳ�ü����и����е����ʣ�
�����صķ���ʽ��C15H14O6?H2O������������C��H��O����Ԫ�أ�
�ʴ��ǣ���1�����ڰ״�Ƭ����Ƭ�Ϸ�һСƬpH��ֽ��������ҺͿ����ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ��ó�����Һ��pH���������������������� ����������̼����
��2��ABC��ȡ�����������ˮ�������۲�����
��3�������ʡ�3��
��������������Ⱦ���ֹ������ע�Ľ������⣬��֮��صĿ���ͳ�Ϊ�п����ȵ�֮һ��

��ϰ��ϵ�д�
�����Ŀ
������������벻����ѧ��
��1�����µĿ������ྻ������ˮ�������Ӫ���������彡��ϢϢ��أ�
�����µĿ������� ��ѡ��������������
�ڽྻ������ˮ�ھ������������� �ķ�����ȥˮ�в��������ʣ�����
����ɫ�غ���ζ��Ȼ��������������������X��һ�ֳ���������ˮ����������ҵ����ȡX�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2NaCl+2X����X�Ļ�ѧʽ�� ��
�۾����Ӫ����Ҫ������ʳ��ʳƷ�е��л�Ӫ���������ࡢ֬���������ʡ�ά���أ�ʳ��ˮ���߲�Ϊ���岹�����Ҫ�л�Ӫ������ ��
��2�������������ճ������г��õĸ�����ϴ����ѡ���ʵ���Ʒ���Եõ����õ���ϴЧ����
������ʹ��ϴ������ϴ�;��ϵ����ۣ�������Ϊ������ ���ܣ�
���������ʿ���ʹ�ý������ϴ���� ������ĸ��ţ���
A������ B������ C��ˮ��
�������������¯��������ϣ����Է���ͼ��ʾ�Ļ�ѧ��Ӧ��ͼ��a���Ļ�ѧʽΪ ��
�ܡ����ձ�ը�Ρ�����ˮ��ֽ�����Na2CO3��H2O2��������ը������ˮ���ټ��������Ľ���飬�۲쵽������ð�������������Ľ���������Ӧ�Ļ�ѧ����ʽΪ ��
�ݹ�ҵ�Ͻ�������Cl2��ͨ���ռ���Һ�п���ȡ����Һ����Ӧ���γ���NaCl��NaClO����Һ���÷�Ӧ�Ļ�ѧ����ʽΪ ��

��1�����µĿ������ྻ������ˮ�������Ӫ���������彡��ϢϢ��أ�
�����µĿ������� ��ѡ��������������
�ڽྻ������ˮ�ھ������������� �ķ�����ȥˮ�в��������ʣ�����
����ɫ�غ���ζ��Ȼ��������������������X��һ�ֳ���������ˮ����������ҵ����ȡX�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2NaCl+2X����X�Ļ�ѧʽ�� ��
�۾����Ӫ����Ҫ������ʳ��ʳƷ�е��л�Ӫ���������ࡢ֬���������ʡ�ά���أ�ʳ��ˮ���߲�Ϊ���岹�����Ҫ�л�Ӫ������ ��
��2�������������ճ������г��õĸ�����ϴ����ѡ���ʵ���Ʒ���Եõ����õ���ϴЧ����
| ���� | ϴ���� | ����� | ¯������ | ���ձ�ը�� | ����Һ |
| ��Ʒ ��ʽ |  |  |  |  |  |
| ��Ч�ɷֻ��� | ��ϴ���� | ���� | �������� | ��̼���� | ���� |
���������ʿ���ʹ�ý������ϴ���� ������ĸ��ţ���
A������ B������ C��ˮ��
�������������¯��������ϣ����Է���ͼ��ʾ�Ļ�ѧ��Ӧ��ͼ��a���Ļ�ѧʽΪ ��
�ܡ����ձ�ը�Ρ�����ˮ��ֽ�����Na2CO3��H2O2��������ը������ˮ���ټ��������Ľ���飬�۲쵽������ð�������������Ľ���������Ӧ�Ļ�ѧ����ʽΪ ��
�ݹ�ҵ�Ͻ�������Cl2��ͨ���ռ���Һ�п���ȡ����Һ����Ӧ���γ���NaCl��NaClO����Һ���÷�Ӧ�Ļ�ѧ����ʽΪ ��

 ���µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
���µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺ ��2012?Ϋ�������µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
��2012?Ϋ�������µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺ N2+4CO2���ڸ÷�Ӧ�л��ϼ۱仯��Ԫ��Ϊ______����дԪ�����ƣ���
N2+4CO2���ڸ÷�Ӧ�л��ϼ۱仯��Ԫ��Ϊ______����дԪ�����ƣ���