��Ŀ����
��7�֣�����һ�������ĩ��������CaCO3��CaO��Na2CO3�е�һ�ֻ�����ɡ�Ϊȷ������ɣ�����������ͼ��ʾ��ʵ�飨����ʵ���������ģ���
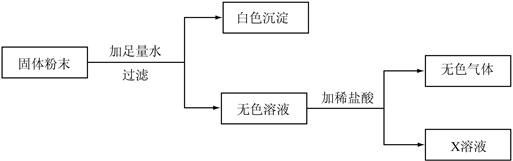
��ش�
��1������ʵ�����ȷ����ɫ������ ��ԭ�����ĩ��һ������ �����Ŀ�������� �֡�
��2����ʵ��ȡ�����ĩ20g�����ɰ�ɫ����10g�����õ�������������Ϊ5.85%��X��Һ200g��ֻ��һ�����ʣ�����X��Һ�����ʵ������� g�������ĩ�� ���ѧʽ����ͬ����ɣ���������С��10g���� ��

��ش�
��1������ʵ�����ȷ����ɫ������ ��ԭ�����ĩ��һ������ �����Ŀ�������� �֡�
��2����ʵ��ȡ�����ĩ20g�����ɰ�ɫ����10g�����õ�������������Ϊ5.85%��X��Һ200g��ֻ��һ�����ʣ�����X��Һ�����ʵ������� g�������ĩ�� ���ѧʽ����ͬ����ɣ���������С��10g���� ��
��7�֣���1��CaCO3����̼��ơ��� Na2CO3����̼���ơ��� 3
��2��11.7����11.7g��Ҳ���֣� CaCO3��CaO��Na2CO3��2�֣��ٴ��֣�
CaCO3��CaO���ٴ��֣�
��2��11.7����11.7g��Ҳ���֣� CaCO3��CaO��Na2CO3��2�֣��ٴ��֣�
CaCO3��CaO���ٴ��֣�
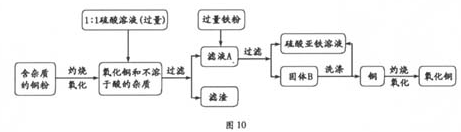
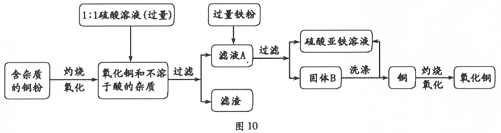
�ѹ����ĩ����ˮ�У��õ���ɫ������������������ʿ����жϸð�ɫ����Ϊ̼��ƣ��õ���ɫ��Һ��ϡ���ᷴӦ������ɫ���壬�����ж�����ɫ��Һ�к���̼���ƣ�����ɫ����Ϊ������̼����ôX�Ϳ������Ȼ��Ƶ���Һ�����˸����ʾ��Ѽ�����Ͽ��Ծݴ˴��⣮
�⣺��1���ڰ�ɫ��ĩ���ܲ�����ɫ��������֪�ð�ɫ����Ӧ��Ϊ̼��ƣ��ֵõ�����ɫ��Һ�ܹ���ϡ���ᷴӦ�������壬����֪���ڹ����ĩ��һ������̼���ƣ������ܵ����Ϊ����̼���ƣ������ƣ� ��̼���ƣ�̼��ƣ� ��̼���ƣ������ƣ�̼��Ƶ����ֿ��ܵ���ɣ�
��2�����ݣ�1���Ľ���֪X��ҺΪ�Ȼ�����Һ������Ϊ��200g��5.85%=11.7g�����������ϻ�ѧ����ʽ��������������ᷴӦ��̼���Ƶ�����Ϊ10.6g�����������жϳ��ù����ĩ�����Ϊ��CaCO3��CaO��Na2CO3 �����������ж�����ɫ��������С��10g����ԭ���������Ϊ�����ƺ�̼��ƣ�����������£�
�⣺��̼���Ƶ�����Ϊx����
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117
x 11.7g
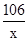 =
=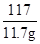
���x=10.6g
����������õ�ֻ�Ǻ����ᷴӦ��̼���ƣ�������Ϊ20g�����Լ����ɫ�����ǹ����ĩ�еģ���ֻ��20g-10.6g=9.4g������Ŀ�еõ��İ�ɫ������������10g��Ҳ����˵��ɫ������������ȫ������ԭ�����ĩ�У�������һ�����Ƿ�Ӧ�����ɵģ��������ƺ�ˮ��Ӧ�������������ƣ������������ֺ�̼���Ʒ�Ӧ������̼��Ƴ������Ӷ������ж��ڻ�����к��������ƣ�
�ʱ����Ϊ����1��CaCO3����̼��ơ����� Na2CO3����̼���ơ����� 3��
��2��11.7�� CaCO3��CaO��Na2CO3��CaCO3��CaO��
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ��������ʵĻ�ѧ����ֱ�ӵó����ۣ�Ȼ������˳�ƻ����ƻ���������м��ƶϣ��ó��������ۣ�
�⣺��1���ڰ�ɫ��ĩ���ܲ�����ɫ��������֪�ð�ɫ����Ӧ��Ϊ̼��ƣ��ֵõ�����ɫ��Һ�ܹ���ϡ���ᷴӦ�������壬����֪���ڹ����ĩ��һ������̼���ƣ������ܵ����Ϊ����̼���ƣ������ƣ� ��̼���ƣ�̼��ƣ� ��̼���ƣ������ƣ�̼��Ƶ����ֿ��ܵ���ɣ�
��2�����ݣ�1���Ľ���֪X��ҺΪ�Ȼ�����Һ������Ϊ��200g��5.85%=11.7g�����������ϻ�ѧ����ʽ��������������ᷴӦ��̼���Ƶ�����Ϊ10.6g�����������жϳ��ù����ĩ�����Ϊ��CaCO3��CaO��Na2CO3 �����������ж�����ɫ��������С��10g����ԭ���������Ϊ�����ƺ�̼��ƣ�����������£�
�⣺��̼���Ƶ�����Ϊx����
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117
x 11.7g
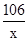 =
=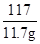
���x=10.6g
����������õ�ֻ�Ǻ����ᷴӦ��̼���ƣ�������Ϊ20g�����Լ����ɫ�����ǹ����ĩ�еģ���ֻ��20g-10.6g=9.4g������Ŀ�еõ��İ�ɫ������������10g��Ҳ����˵��ɫ������������ȫ������ԭ�����ĩ�У�������һ�����Ƿ�Ӧ�����ɵģ��������ƺ�ˮ��Ӧ�������������ƣ������������ֺ�̼���Ʒ�Ӧ������̼��Ƴ������Ӷ������ж��ڻ�����к��������ƣ�
�ʱ����Ϊ����1��CaCO3����̼��ơ����� Na2CO3����̼���ơ����� 3��
��2��11.7�� CaCO3��CaO��Na2CO3��CaCO3��CaO��
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ��������ʵĻ�ѧ����ֱ�ӵó����ۣ�Ȼ������˳�ƻ����ƻ���������м��ƶϣ��ó��������ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ