��Ŀ����
����Ŀ��ijͬѧΪ̽�����Ͻ������������������Ⱥ�������Ĵ�ʵ�飨�����������ɷֲ���ϡ���ᷴӦ����ʵ���������±������ݸ�ͬѧ��ʵ�飬�Իش��������⣺
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
��ȡ�Ͻ�������Mg | 10 | 10 | 20 | 30 |
����ϡ����������Mg | 100 | 120 | 80 | X |
���������������Mg | 0.2 | 0.2 | 0.2 | Y |
��1���ϱ����Ĵ�ʵ���кϽ������ǡ����ϡ������ȫ��Ӧ��������X=__Y=__��
��2����ͭ���Ͻ����������������Ƕ���__��
��3������ϡ����������������Ϊ����______�������������0.1%����
���𰸡�240 0.6 56�� 12.3��
��������
��1���ɵ�һ��ʵ��͵ڶ���ʵ���֪������0.2g������Ҫ�Ͻ������Ϊ10g���ɵ�һ��ʵ��͵�����ʵ���֪����0.2g������Ҫϡ���������Ϊ80g����10g�Ͻ���80gϡ����ǡ����ȫ��Ӧ����30g�Ͻ�����240gϡ����ǡ����ȫ��Ӧ����0.6g���壬����240��0.6��
�⣺��ͭ���Ͻ���������������Ϊx��ϡ����������������Ϊy
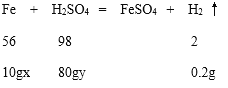
56��2=10gx��0.2g x=56��
98��2=80gy��0.2g y��12.3��
�𣺣�2����ͭ���Ͻ�����������������56����
��3������ϡ����������������Ϊ12.3��.

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����A��B��������ѡһ����������������𣬰�A�Ʒ֡�
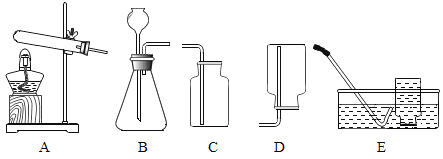
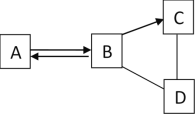
A | B |
��1��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ��________�� ��2����װ��A��E��ȡ������ѡ��װ��E�ռ�������ԭ����________�� | ��1��ʵ������ȡ������̼�Ļ�ѧ����ʽ��________ ��2����װ��B��C��ȡ������̼�����������̼���ռ����IJ�����________�� |
����Ŀ��ijͬѧ��CaH2���Ʊ������ʽ���̽����
���Ķ����ϣ�����H2��Ƽ����Ƶ�CaH2������ˮ�����������ҷ�Ӧ����һ�ּ��һ������
��CaH2Ҫ�ܷⱣ�棬��ˮ��ӦҲ����һ�ּ��һ������
��CaH2���Ʊ�����Ƶ���ȡװ����ͼ��ʾ��
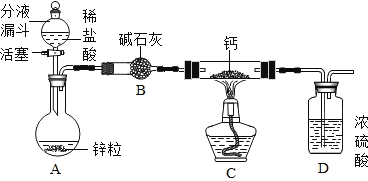
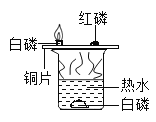
��1��װ��D��������_____��
��2���Ʊ�CaH2ʵ�������ȡ�������С�ļ���ˮ�У��۲������ݲ���������Һ�е���ʯ����Һ����_____ɫ����ͬѧ�ݴ��жϣ�ʵ����ȷ���⻯�����ɣ�����ͬѧ������۲�һ����ȷ��ԭ����_____��
��CaH2������̽����ȡ���������Ƶõ�CaH2 ��Ʒ���뵽������̼������Һ�У������������ݣ����ˣ��õ���������Һ�������������ijɷ���̼��ơ���ͬѧ���������µ�̽����
��3����ȼ���������壬���浭��ɫ����ȼ�ղ���ͨ�����ʯ��ˮ�У��������������Ϊ______��д��ѧʽ����
��4������Һ�����ʵijɷ��������²²Ⲣ����ʵ�飺
����һ��NaOH
�������NaOH��Ca(OH)2
��������NaOH��Na2CO3
�����ģ�NaOH��Na2CO3��Ca(OH)2
�������ۣ����һ����Ϊ�����IJ����������û�ѧ����ʽ˵��ԭ��________
��ʵ����֤��
ʵ�� | ���� | ���� |
ʵ��һ��ȡ��Һ�������е������� Na2CO3��Һ | ______ | ����������� |
ʵ�������ȡ��Һ�������м�������ϡ���� | ______ | ���������� |
������������
��5��ȡ�Ƶõ�CaH2��Ʒ1g������Ϊδ��Ӧ���Ca�����뵽������Na2CO3��Һ�У���ַ�Ӧ���ˡ�ϴ�ӡ�����Ƶ�CaCO3����Ϊ2.4g�������Ʒ��CaH2����������Ϊ______��